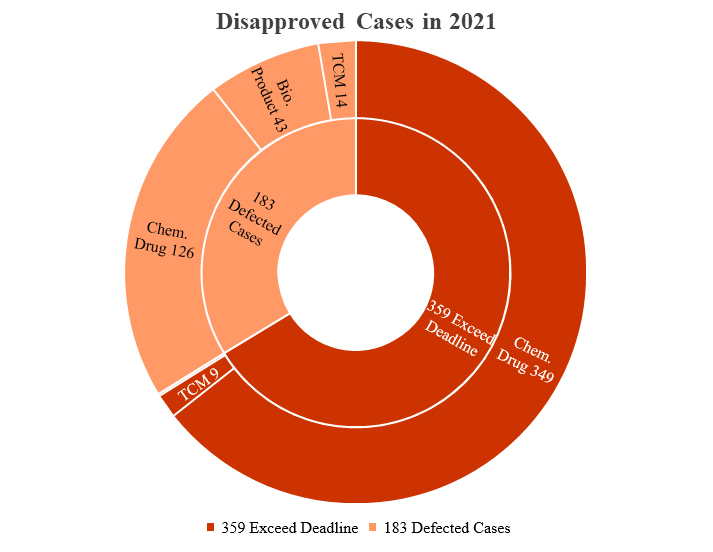
In 2021, China’s Center for Disease Evaluation (CDE) has rejected 542 drug registration applications. 359 rejected applications were due to failure of submitting supplementary dossiers in time (hereinafter “Exceed Deadline”), accounting for 66.3% of all rejected applications. The overdue cases included applications of 9 Traditional Chinese Medicine (TCM), 349 chemical drugs, and 1 biological product.
The remaining 183 applications (hereinafter “Defected Cases”) were rejected for reasons related to safety, efficacy, quality and others, including 14 TCMs, 126 chemical drugs, and 43 biological products (see the following chart).
- Growing Number of Approvals for Surging Registration Applications
- Insufficient Information and Feasibility
This problem exists mainly in the early phase of developing drugs (IND phase) and certain generic drugs as well as supplementary applications.
Mostly in the following aspects:
- Unclear clinical position of drug R&D
- Unreasonable selection of indication
- Unreasonable choice of dosage form or route of administration,
- Ineffective drug suggested by existing research data,
- Ambiguous action target and mechanism, etc.
- Drug Efficacy Issues
- The clinical study data provided could not demonstrate efficacy of the drug.
- Issues related to clinical trial protocol or study quality control, resulting in the invalidation of the effectiveness of the tested drug indication.
- The results of BE test of generic drugs showed no equivalency to the RLD.
- Lack of clinical data for Chinese ethnic group
- Drug Safety Issues
- Early IND phase findings suggest that the toxicity is significant, or the safety risks are too high to justify further clinical developments.
- Issues with the pre-clinical safety study methods or study quality control issues, or insufficient study data to support subsequent clinical development.
- The existing clinical study data shows serious adverse reactions, and the ratio of clinical benefit versus risk is unreasonable.
- Lack of safety clinical data in Chinese Ethnic groups
- Quality Controllability
Serious defects in pharmaceutical research:
- Inconsistency of quality of RLD
- Inconsistency of study samples tested at each development stage
- Failure to meet the Chinese technical requirements for marketing of generic drugs
- Failure to use APIs of legitimate origin as required
- Inconformity of testing samples according to the Chinese regulations
- Regulatory Compliance
A very significant reason for rejecting drug application is due to regulatory gaps and failure to comply with China NMPA requirements including but not limited to the following three aspects:
- Data Gaps according to the Chinese requirements
- Major defects affecting product quality found
- Disqualification during sampling inspection
- Other problems
- Failure to provide the necessary data or supplementary materials as requested by CDE.
- Lack of necessary contents found during technical review
- Insufficient Information and Feasibility
- Suggestions
- Comply with China Regulatory Requirements
- It is crucial to submit the required materials in time and to catch up with all deadlines.
- It is advised to conduct thorough regulatory compliance assessments and gap analyses of the dossiers so as to minimize the probability of being disapproved.
- To attach sufficient importance to basis of drug development
- Drug development and registration should be based on clinical needs and size of the Chinese market. Special attention should be paid to solving unmet clinical needs.
- Clinical value should be put in leading position. Full attention should be paid to the advantages of similar innovative drugs development, and to avoiding grouping, low-level and repetitive innovation.
- Comply with China Regulatory Requirements
Authorized Agent in China
Accestra Consulting has successfully helped overseas manufacturers to register medicines in China. We can be your trusted appointed agent by letter of authorization (LoA).
Contact Us
For information or request for proposal, please email: info@accestra.com
We would be more than happy to arrange a teleconference call to share an overview of the China drug registration requirements, costs, and timelines. Email us to arrange a date and time to call (Info@accestra.com).








