Drug Master File (DMF) Filing in China
APIs, Excipients, Packaging
Who needs to submit a DMF File in China
An overseas manufacturer of an Active Pharmaceutical Ingredient (API), a pharmaceutical excipient or packaging material must apply for marketing authorization for the product in China through DMF filing.
This is according to the regulatory requirements for DMF filing in China:
- The Drug Administration Law of the People’s Republic of China (2019 Revision),
- The Provisions for Drug Registration (2020 SAMR Decree No. 27).
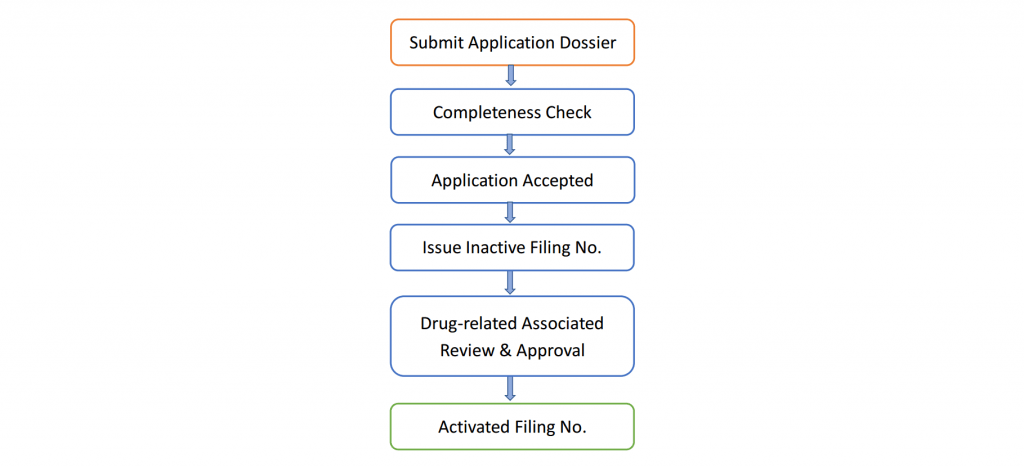
Once an application for DMF filing is submitted to the Center for Drug Evaluation (CDE), they will arrange a technical review for the product in combination with its related finished dosage form (Drug-related Associated Review).
A filing number will be issued for every accepted application by CDE and the general product information will be published online.
View a summary of the workflow of China DMF filing below (Fig. 1):