Cosmetic product in China is defined as “a kind of daily-used chemical product intended to be applied on the surface of human body (skin, hair, nails, lips, etc.) by rubbing, spraying or otherwise similar ways for the purpose of cleansing, protecting, beautifying and altering the appearance”.
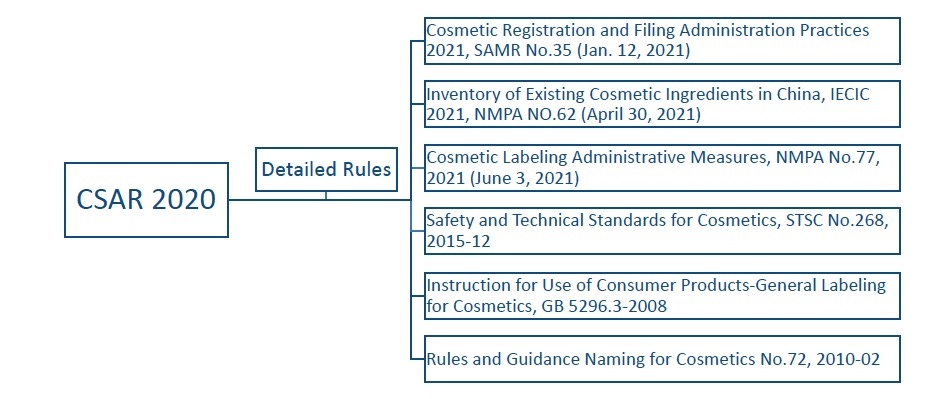
√ Pillar cosmetic regulation: Cosmetic Supervision and Administration Regulation (CSAR 2020, State Council Decree No.727): officially released in June 2020 and came into force since January 1st, 2021.
√ Cosmetic Registration and Filing Administration Practices 2021 (SAMR No.35): officially released in January 2021.
√ IECIC 2021 (NMPA No.62) (including reference data, i.e., highest history usage for rinse-off and leave-on products): officially released in April 2021.
√ Cosmetic Labelling Administrative Measures (NMPA No.77): officially released in June 2021.
√ Safety and Technical Standards for Cosmetics, STSC No.268, 2015-12
√ ……
CSAR’s relevant practices for cosmetic registration and filing has been taken into effect officially from 1st May 2021 on. And more detailed rules drafts are under public consultation, like guidance for cosmetic efficacy evaluation, cosmetic labeling administrative measures, etc.
Various subsidiary rules, standards and guidance documents together with the pillar regulation has formed a complex regulation system for cosmetic management in China.
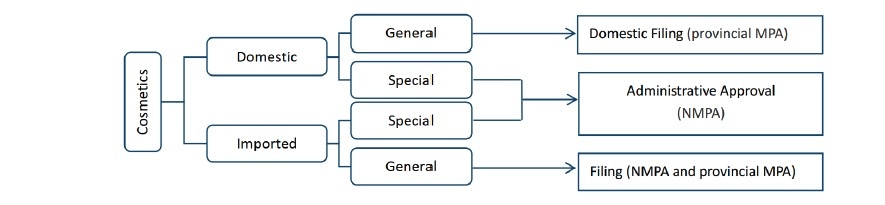
In China, cosmetics is divided as two groups:
◊ Special cosmetics refer to 7 types: hair dye, hair perm, anti-spot, whitening, UV protection (SPF/PA), anti-hair loss/alopecia and new functions.
◊ General cosmetics: the others without the above functions, like moisturizing cream, shampoo, shower gel, lipstick, etc.
Before importing or selling cosmetics, it is mandatory to apply pre-market approval or filing from National Medical Products Administration (NMPA) or provincial Medical Products Administration (provincial MPA).
Generally, all cosmetic products should be produced/imported only after getting the pre-market administrative approval via NMPA or provincial MPA.
While overseas manufacturer shall authorize an independent legal entity to play the role of RP (Responsible Person), who is responsible for cosmetic registration/filing.
Accestra cosmetics experts are experienced in all types of cometic registration and filing procedure and relevant regulatory consulting advisory. We provide tailored solutions based on your products and business needs.