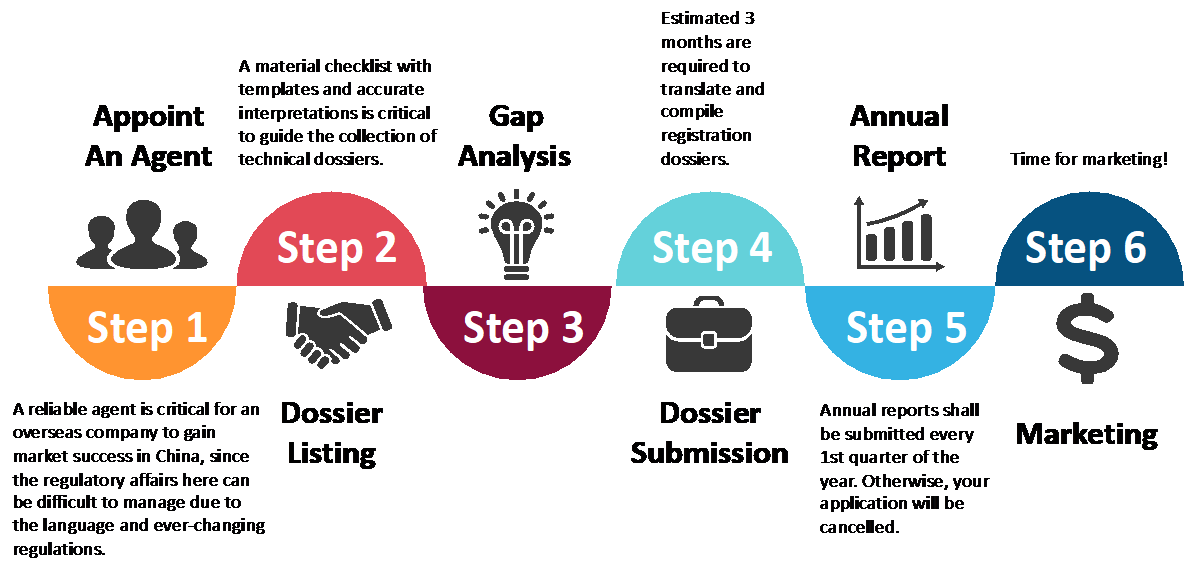
Pharmaceutical regulatory compliance is a relatively time- and resource-consuming work for pharmaceutical companies, especially when they are exploring a novel market with limited knowledge of its regulatory concepts. Meanwhile, the fast growing global market requires companies to have breakthrough solutions in order to expedite their market access under stringent supervision. Therefore, more and more pharmaceutical companies choose to outsource regulatory compliance to professional service providers, such as a consulting company like ACCESTRA. We have not only abundant experience in regulatory affairs but also close relationships with the competent authorities in China. With our help, pharmaceutical companies could centralize their limited resources for research and development in order to have a boost in growth and gain success more rapidly on the market.
ACCESTRA has regulatory specialists who dedicate themselves to assist our clients in designing the optimum market access strategy, exploring the best compliance practice for each actual circumstance, and providing practical advices on compliance management for pharmaceutical or medical products. We provide a wide range of regulatory & technical consulting services which cover from the pre-IND stage of pharmaceutical development to NDA/ANDA registration, DMF filing and technology transfer supports.