How to qualify for a clinical trial exemption
- An evaluation of the existing clinical data conducted overseas,
- The drug clinical need and urgency in China, which can you can discuss with CDE before the Investigational New Drug (IND) application in a pre-IND meeting.
Top 10 Questions to China’s clinical trial exemption and IND application
It depends. According to the Technical Guidelines for Acceptance of Drug Foreign Clinical Data (National Medical Products Administration [2018] Decree No.52), the acceptance of drug foreign clinical data depends on the data quality, drug efficacy, safety, and ethnic factors. Acceptance will be possible if your data is:
- Authentic, reliable, in compliance with ICH guidelines for Good Clinical Practice (GCP) and Chinese regulatory requirements for on-site inspection and audit.
- Sufficient for the evaluation of drug efficacy and safety for the target indication.
- Sufficient to demonstrate that no impact of ethnic factors has been identified on drug efficacy and safety.
Need a compliance assessment of your data? Please contact us on info@accestra.com
If your clinical data fulfills all the criteria for acceptance and your drug has been approved outside of China (in the US, EU, Japan etc.) for an urgent or unmet clinical need, such as a life-threatening or rare disease, according to the Technical Requirements for Clinical Trials on Drugs Marketed Overseas but Unavailable in China (Center for Drug Evaluation [2020] Decree No.29), clinical trials could be exempted in China.
If your clinical data is evaluated to be insufficient by China CDE, clinical trials will be required in China in forms of bridging studies or comprehensive clinical trials depending on the overall data quality.
You can apply for a pre-IND meeting with CDE to evaluate your clinical data and discuss the feasibility of exemption.
If no exemption is possible, you can provide CDE with a strategy for clinical studies in China and ask for their opinions.
Not yet. You need to register your clinical trials on the Platform for Drug Clinical Trials Registration and Information Publishing with China CDE for government supervision and public review (Fig. 1).
Fig.1 Platform for Drug Clinical Trials Registration and Information Publishing
Yes. All clinical studies in China need to be registered on the platform including:
- Phase I-IV clinical trials,
- bridging studies,
- pharmacokinetics (PK)
- pharmacodynamics (PD),
- bioavailability (BA),
- and bioequivalence (BE) studies.
- Applicant name,
- Company name and address,
- Drug name,
- Indication,
- IND approval number,
- Approval date.
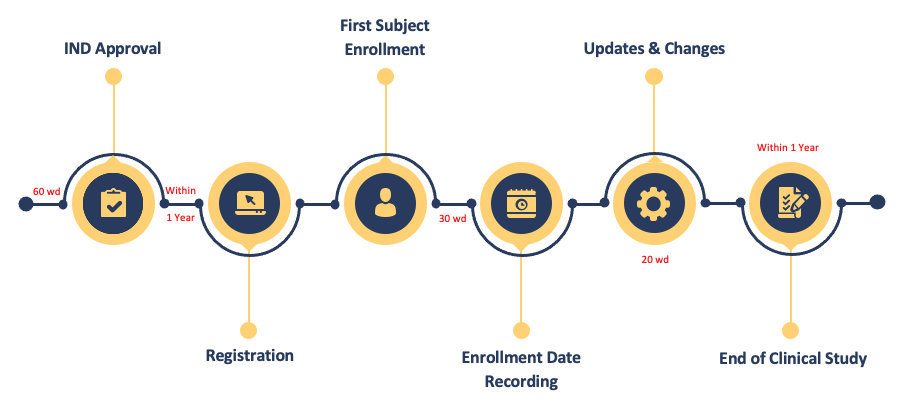
The timeline of clinical trial registration is as follows (Fig. 2).
-
Fig.2 Timeline of Drug Clinical Trials Registration
IND approval: An IND approval is issued by default in 60 wd by CDE.
- Registration: Register your clinical study on the above-mentioned platform within 1 year after IND approval so that the relating data will be published for public review before the first subject is enrolled.
- First Subject Enrollment: The first subject enrolls in the study.
- Enrollment Date Recording: Record the enrollment date on the platform within 30 workdays.
- Updates & Changes: If any updates or changes during the study, submit the data within 20 workdays on the platform.
- End of Clinical Study: After the study ends, update the results on the platform within 1 year.
CDE will only publish part of your registration data on the platform for public review without disclosing confidential information.
The data will be published in 2 weeks each time you submit updates to CDE.