In 2019, the Center for Drug Evaluation (CDE) in China approved a large number of registrations for drug products as well as drug-device combinations.
The majority of drug registrations required technical review and approval, whereas the others required only administrative approval. The number of different registrations approved by China CDE in 2019 is listed in the table below (Table 1).
| REGISTRATION TYPE | NUMBER OF REGISTRATIONS |
|---|---|
| Drug Products | 8077 |
| Technical Review and Approval | 6199 |
| Administrative Approval | 1878 |
| Drug-device Combinations | 5 |
| TOTAL NUMBER | 8082 |
Table 1. Number of Drug Registrations Approved in 2019 by CDE.
1. Overall Summary
The diagram below (Fig. 1) compares registration amounts among different drug products from 2016 to 2019. 80.2% of drug registrations approved in 2019 were for chemical drugs, which had 6475 registrations.
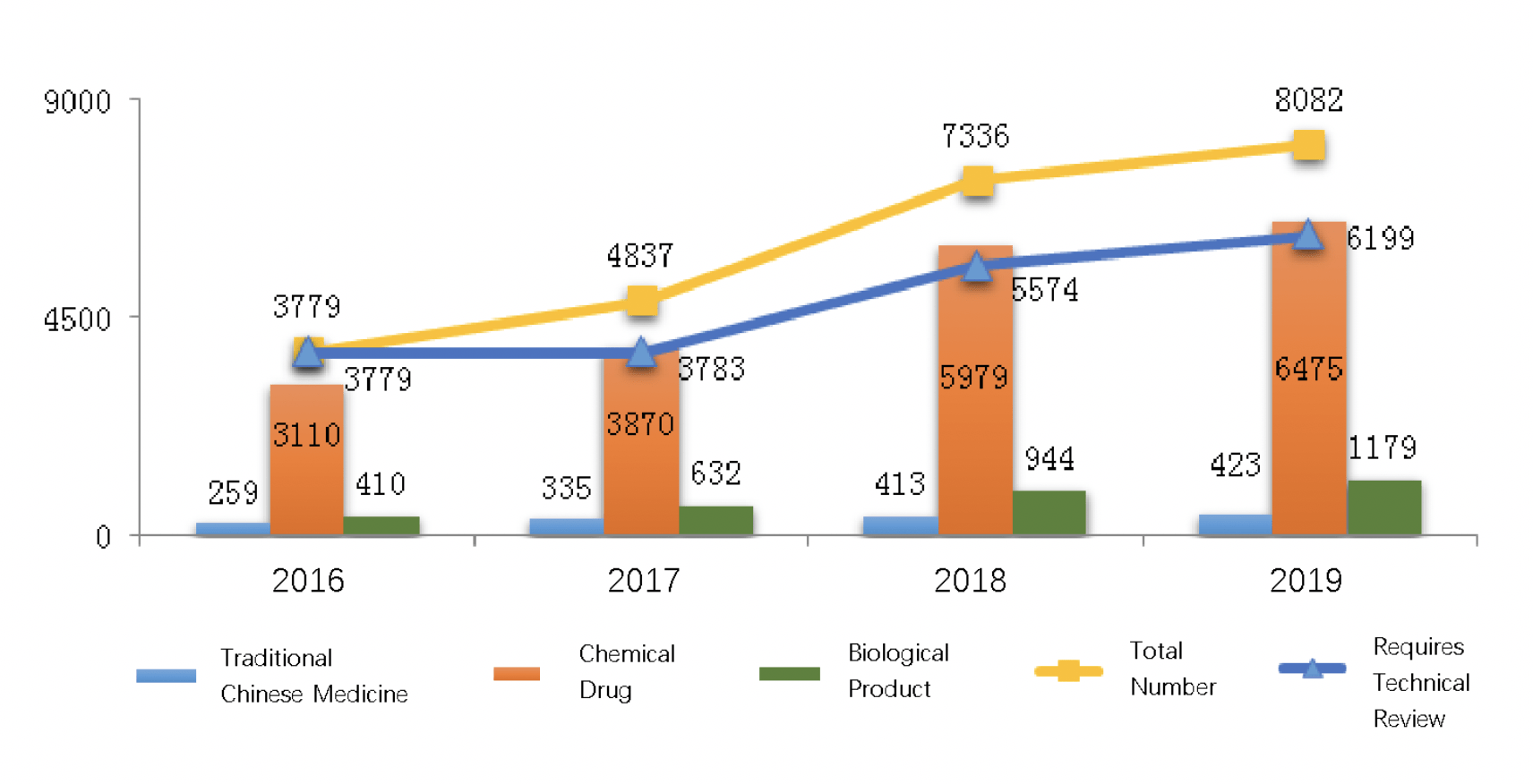
The diagram below (Fig. 2) shows the number of drug registrations approved through technical review from 2016 to 2019. In 2019, a total of 6199 drug registrations were approved through technical review, an increase of 11.21% over last year.
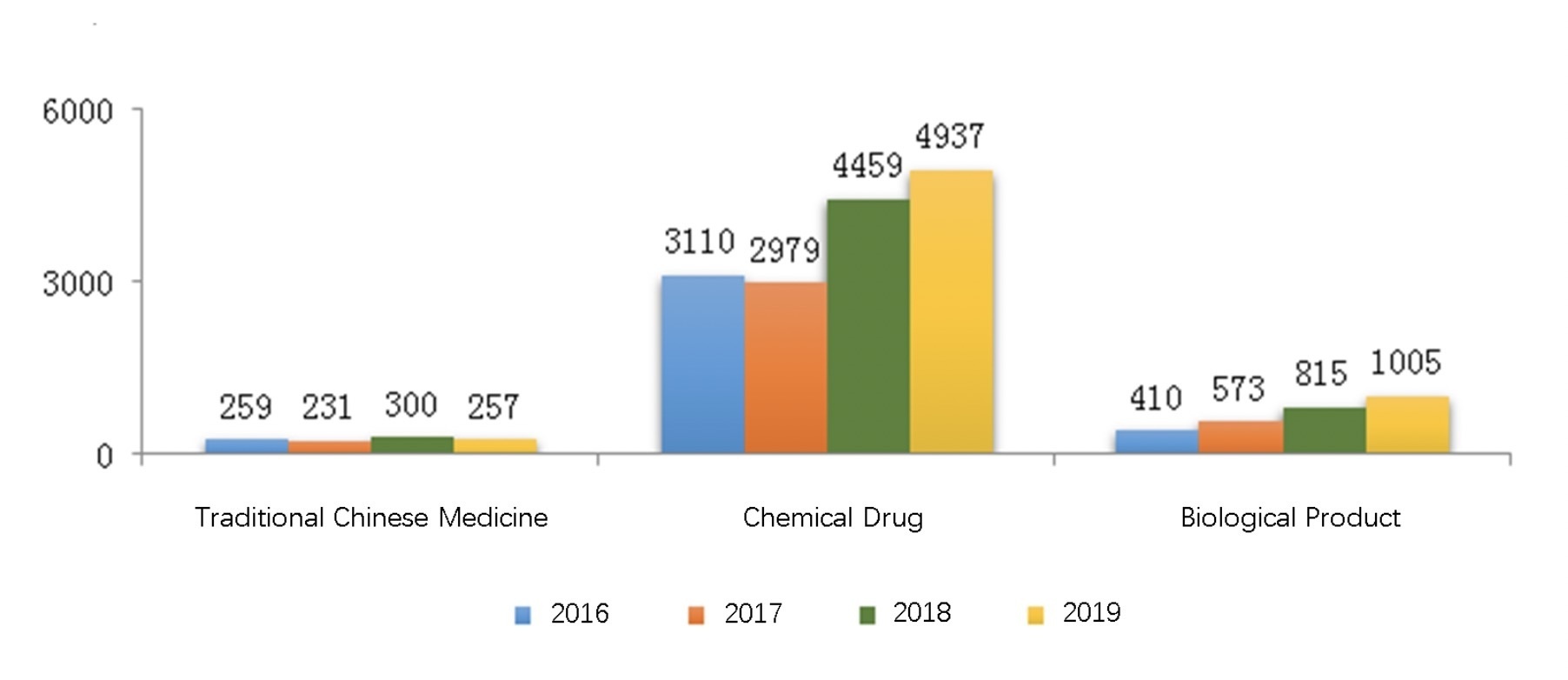
| DRUG CLASSIFICATION | NUMBER OF REGISTRATIONS | % OF TOTAL DRUG REGISTRATIONS | INCREASE OR DECREASE OVER 2018 |
|---|---|---|---|
| Chemical Drug | 4937 | 79.64% | 10.72% |
| Traditional Chinese Medicine | 257 | 4.15% | -14.33% |
| Biological Product | 1005 | 16.21 | 23.30% |
| TOTAL | 6199 | 100% | 11.21% |
Table 2. Number of Drug Registrations Approved through Tenchical Review in 2019.
| REGISTRATION CLASSIFICATION | NUMBER OF DRUG CATEGORIES | INCREASE OR DECREASE OVER 2018 |
|---|---|---|
| IND | 302 | 26.40% |
| NDA | 17 | -32.00% |
| TOTAL | 319 | 21% |
Table 3. Number of Drug Categories of Innovative New Drugs Approved in 2019.
2. Domestic Innovative New Drugs
| CLASSIFICATION | NUMBER OF REGISTRATIONS | NUMBER OF DRUG CATEGORIES |
|---|---|---|
| DRUG CLASSIFICATION | ||
| Chemical Drug | 401 | 144 |
| Biological Product | 127 | 100 |
| REGISTRATION CLASSIFICATION | ||
| IND | 503 | 228 |
| NDA | 25 | 16 |
| TOTAL | 528 | 244 |
Table 4. Number of Registrations and Categories of Domestic Innovative New Drugs Approved in 2019.
3. Imported Innovative New Drugs & Original Drugs
The table below (Table 5) shows the number of drug registrations as well as categories of imported innovative new drugs and original drugs respectively. The indications of these drugs are mainly cancers, endocrine and neurological disorders.
| REGISTRATION CLASSIFICATION | NUMBER OF REGISTRATIONS | NUMBER OF DRUG CATEGORIES |
|---|---|---|
| Class 1 Innovative New Drubs | 172 | 75 |
| Class 5.1 Original Drugs | 157 | 92 |
| TOTAL | 329 | 167 |
Table 5. Number of Registrations and Categories of Imported Innovative New Drugs & Original Drugs Approved in 2019.
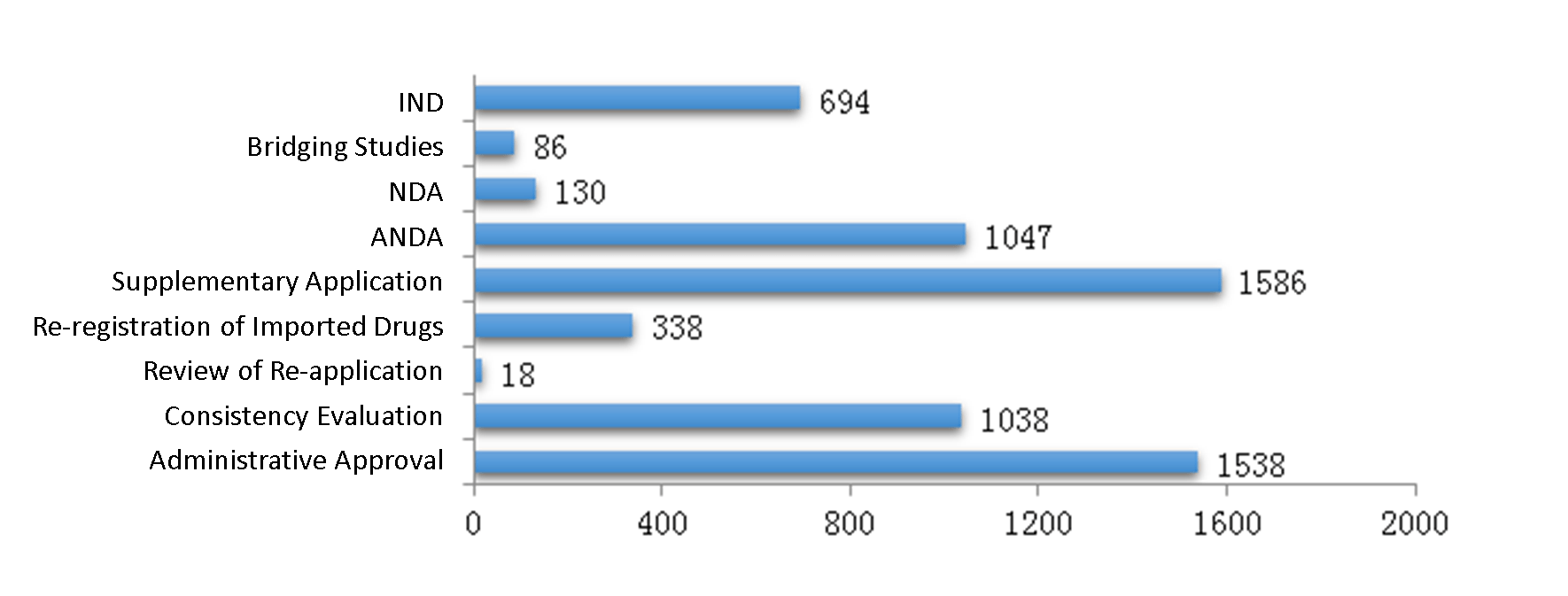
4. Analysis of Drug Registrations
4.1 Analysis of Chemical Drug Registrations
- INDs,
- Bridging studies,
- NDAs,
- ANDAs,
- Supplementary applications,
- Re-registrations of imported drugs,
- Review of re-applications,
- Consistency evaluations,
- Administrative approvals.
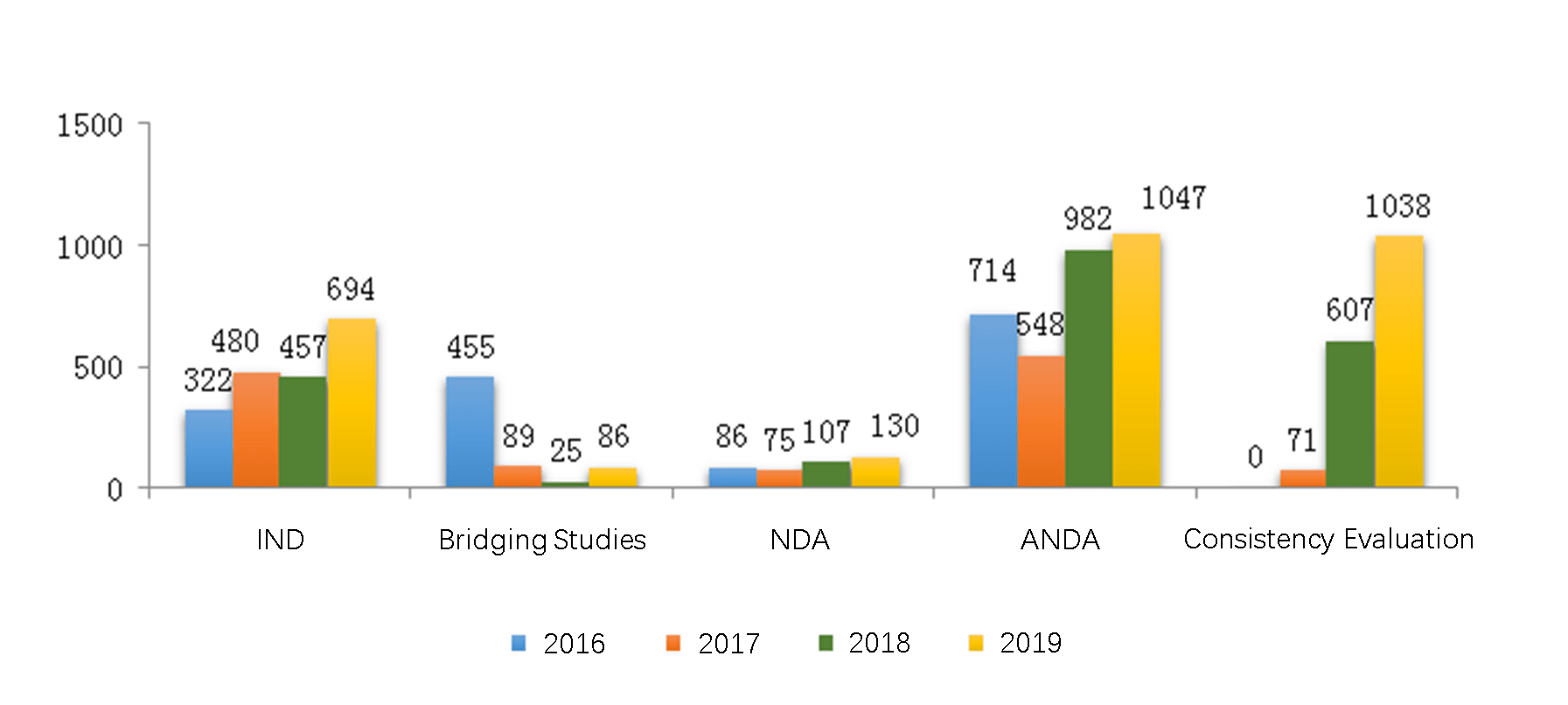
4.1.1 Analysis of IND and NDA Registrations
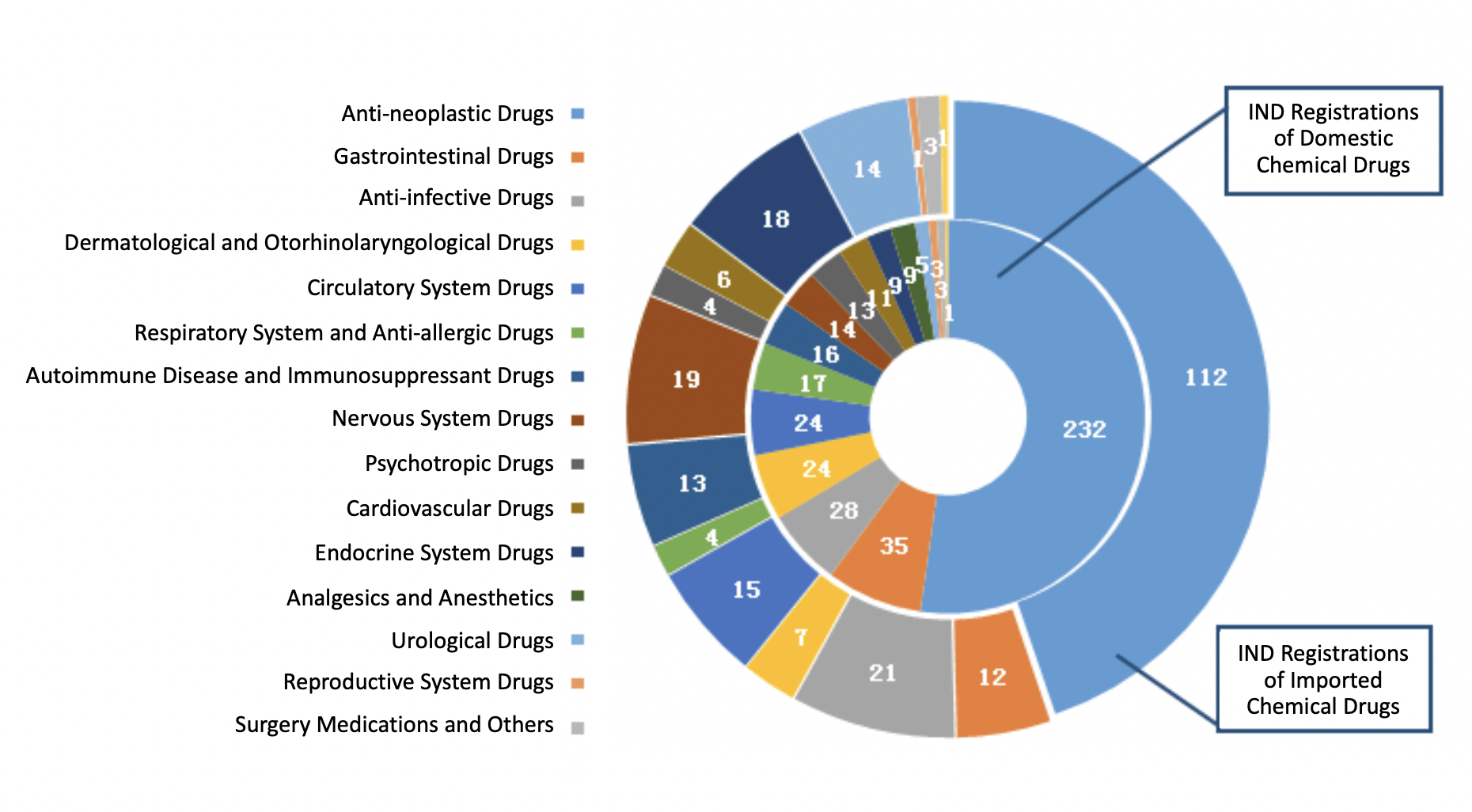
In 2019, CDE approved 444 IND registrations for domestic chemical drugs and 250 IND registrations for imported chemical drugs.
The indications of domestic INDs are mainly cancers, gastrointestinal disorders, and infectious diseases, whereas the indications of imported INDs are mainly cancers, infectious diseases, and neurological disorders.
The diagram below (Fig. 5) shows the number of approved IND registrations for different indications of both domestic and imported chemical drugs in 2019.
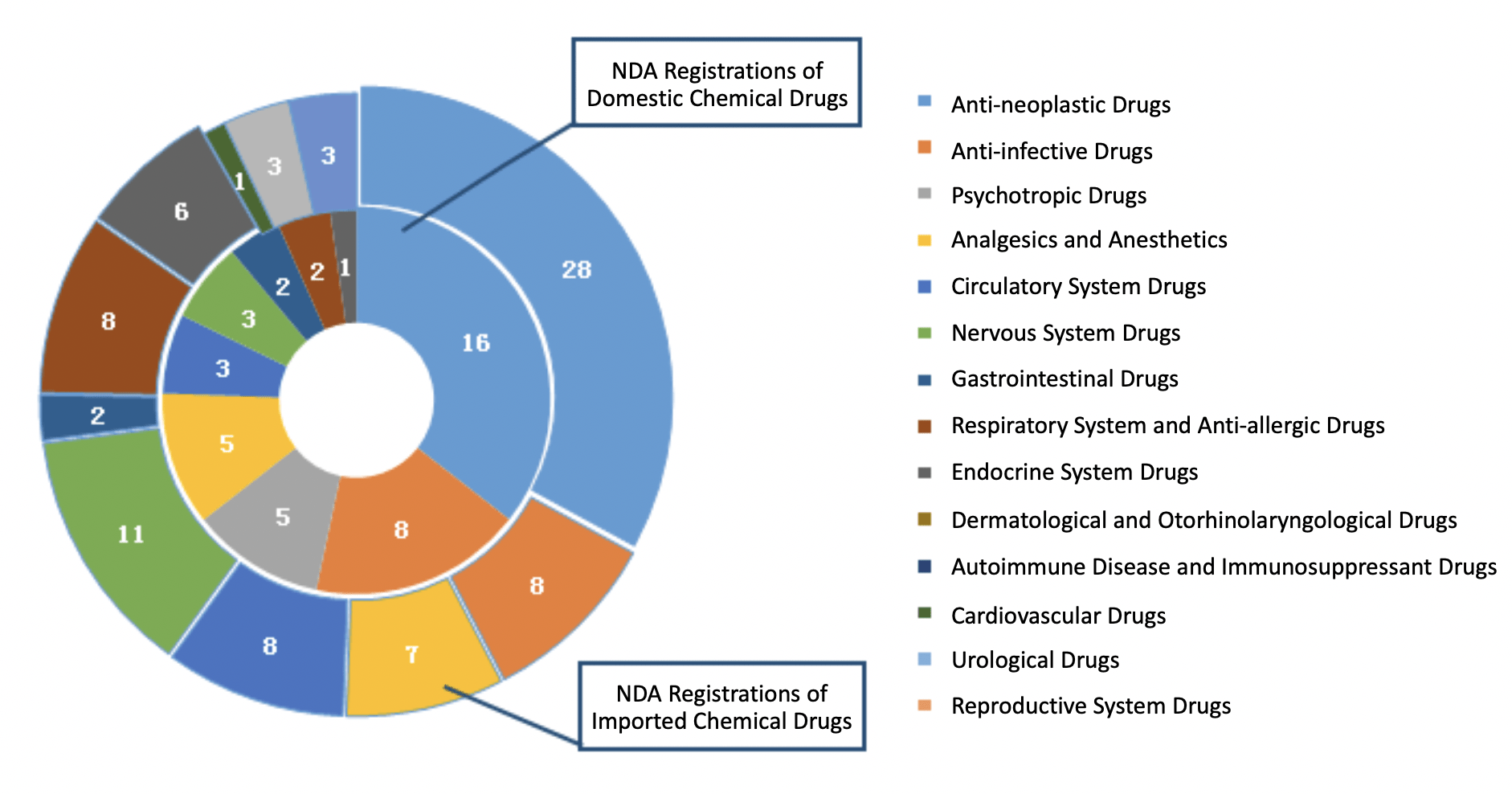
In 2019, CDE approved 45 NDA registrations for domestic chemical drugs and 85 NDA registrations for imported chemical drugs.
The indications of domestic NDAs are mainly cancers and infectious diseases, whereas the indications of imported NDAs are mainly cancers and neurological disorders.
The diagram below (Fig. 6) shows the number of approved NDA registrations for different indications of both domestic and imported chemical drugs in 2019.
4.1.2 Analysis of Innovative New (Chemical) Drug Registrations
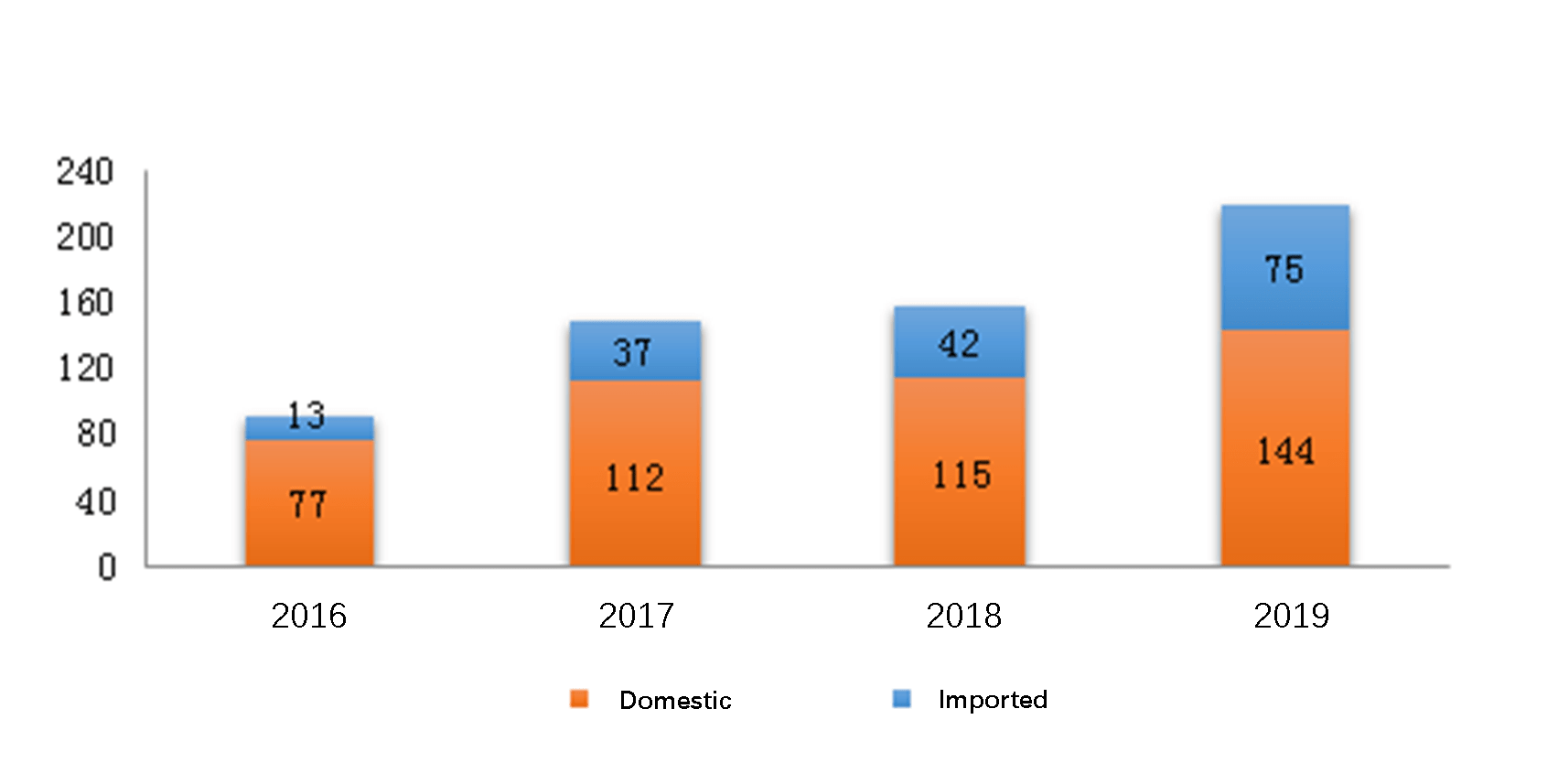
In 2019, CDE approved 573 innovative new drug registrations for chemical drugs under registration Class 1, which covered 219 drug categories.
The table below (Table 6) shows the number of chemical drug categories of IND and NDA registrations respectively.
| REGISTRATION CLASSIFICATION (CHEMICAL DRUGS) |
NUMBER OF DRUG CATEGORIES | INCREASE OR DECREASE OVER 2018 |
|---|---|---|
| IND | 206 | 46.10% |
| NDA | 13 | -18.75% |
| TOTAL | 219 | 39.50% |
Table 6. Number of Drug Categories of IND/NDA Registration Approved in 2019.
The diagram below (Fig. 7) shows the number of approved innovative new drug registrations for domestic and imported chemical drugs respectively from 2016 to 2019.
4.2 Analysis of Traditional Chinese Medicine Registrations
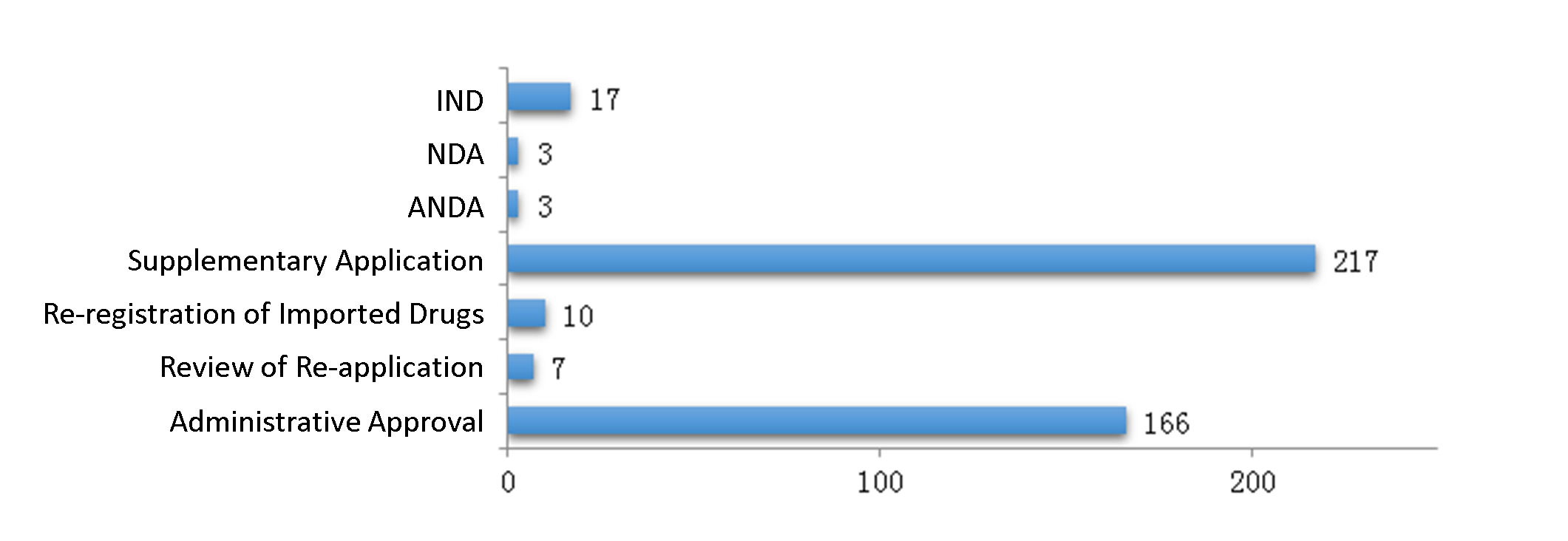
In 2019, CDE approved 423 traditional Chinese medicine registrations, including 17 IND registrations, 3 NDA registrations and 3 ANDA registrations.
The diagram below (Fig. 8) shows the number of traditional Chinese medicine registrations approved for all application types in 2019.
For new or generic traditional Chinese medicines, the number of registrations approved from 2016 to 2019 is shown in another diagram below (Fig. 9) respectively for INDs, NDAs, and ANDAs.
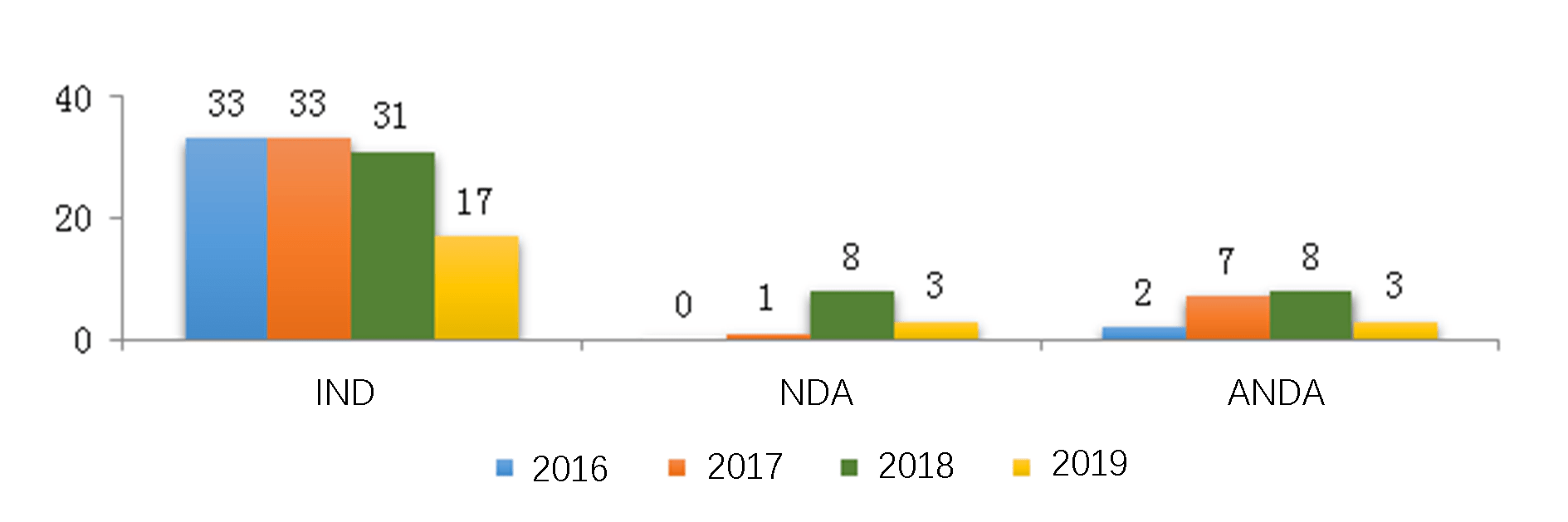
For innovative new traditional Chinese medicines, 17 IND registrations were approved by CDE in 2019, which were mainly indicated for the treatment of gastrointestinal, respiratory and orthopedic disorders.
4.3 Analysis of Biological Product Registrations
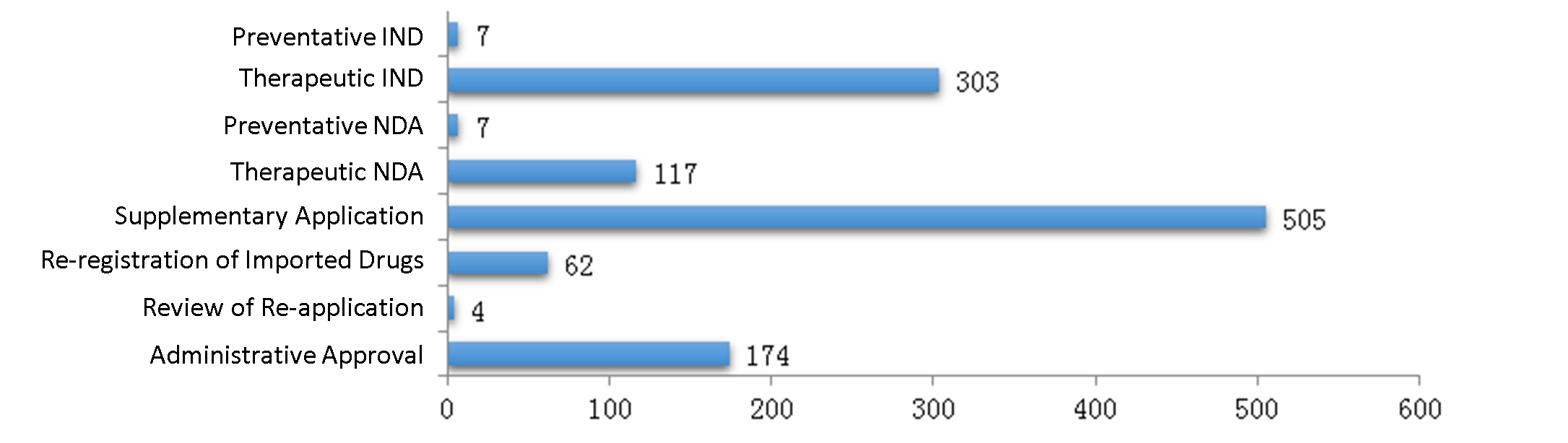
In 2019, CDE approved 1179 biological product registrations, including 310 IND registrations and 124 NDA registrations.
IND registrations had an increase of 4% over the last year, and NDA registrations had an increase of 45.9%.
The diagram below (Fig. 10) shows the number of biological product registrations approved for all application types in 2019. For new or generic biological products, the number of registrations approved from 2016 to 2019 is shown in another diagram below (Fig. 11) respectively for INDs and NDAs (preventative or therapeutic).
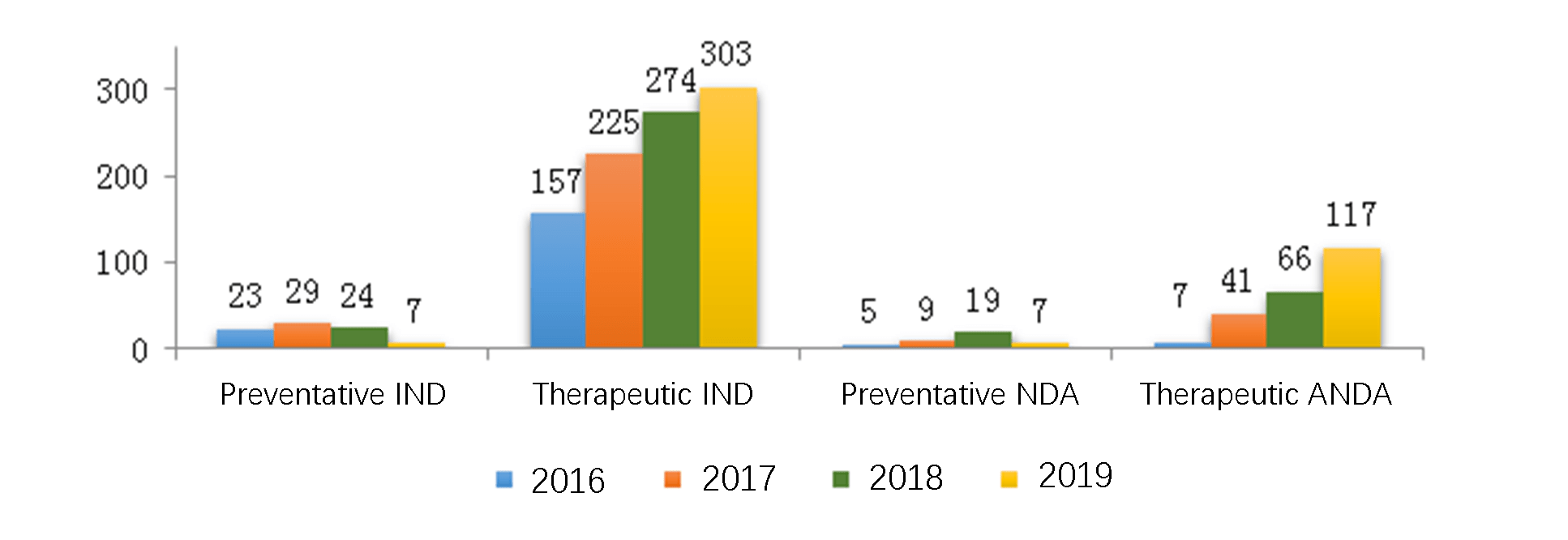
4.3.1 Analysis of Innovative New Biological Product Registrations
In 2019, CDE approved 127 innovative new drug registrations for biological products under registration Class 1, including 2 preventative and 125 therapeutic biological products.
The table below (Table 7) shows the number of drug registrations as well as categories of innovative new biological products respectively for INDs and NDAs.
| REGISTRATION CLASSIFICATION | NUMBER OF REGISTRATIONS | NUMBER OF DRUG CATEGORIES | INCREASE OR DECREASE OVER 2018 |
|---|---|---|---|
| IND | 121 | 96 | 9 |
| NDA | 6 | 4 | -5 |
| TOTAL | 127 | 100 | 4 |
Table 7. Number of Registration and Categories of Innovative New Biological Products Approved in 2019.
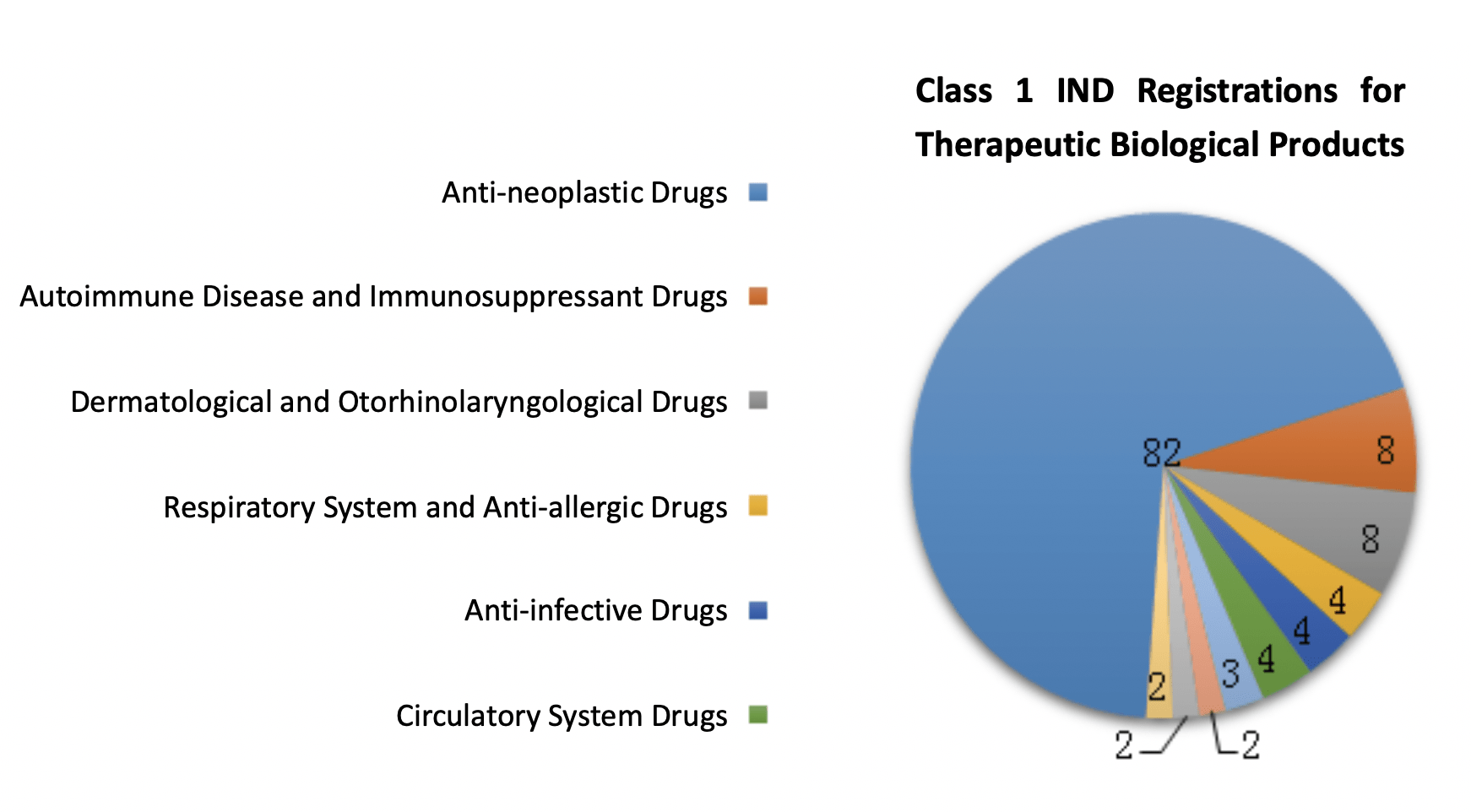
119 therapeutic biological products were approved for IND registrations under registration Class 1.
These products covered 95 drug categories and were mainly indicated for the treatment of cancers.
The diagram below (Fig. 12) shows the number of approved IND registrations for different indications of therapeutic biological products in 2019.
Accestra Consulting provides services for:
- China Regulatory Affairs Outsourcing for Drugs/Pharmaceutical,
- Food and Feed products for China NMPA (Formerly CFDA),
- Regulatory approval including product registration for China Investigational New Drug (IND),
- China New Drug Application (NDA),
- China Drug Master File (DMF) for APIs,
- Excipient and Packaging Materials,
- ANDA,
- eCTD submission and more.
For queries, please contact us at: info@accestra.com