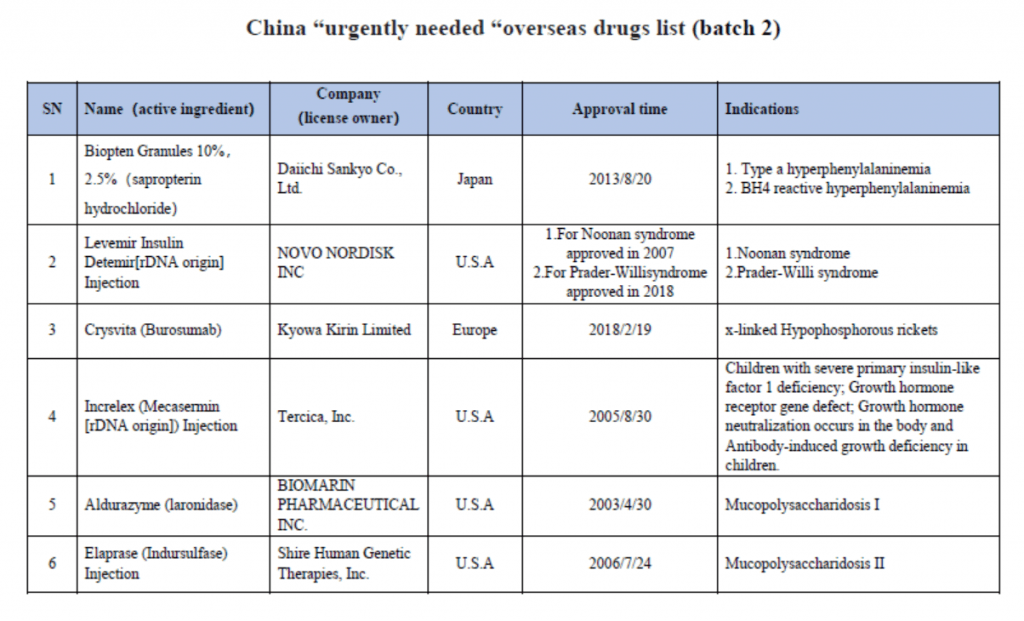
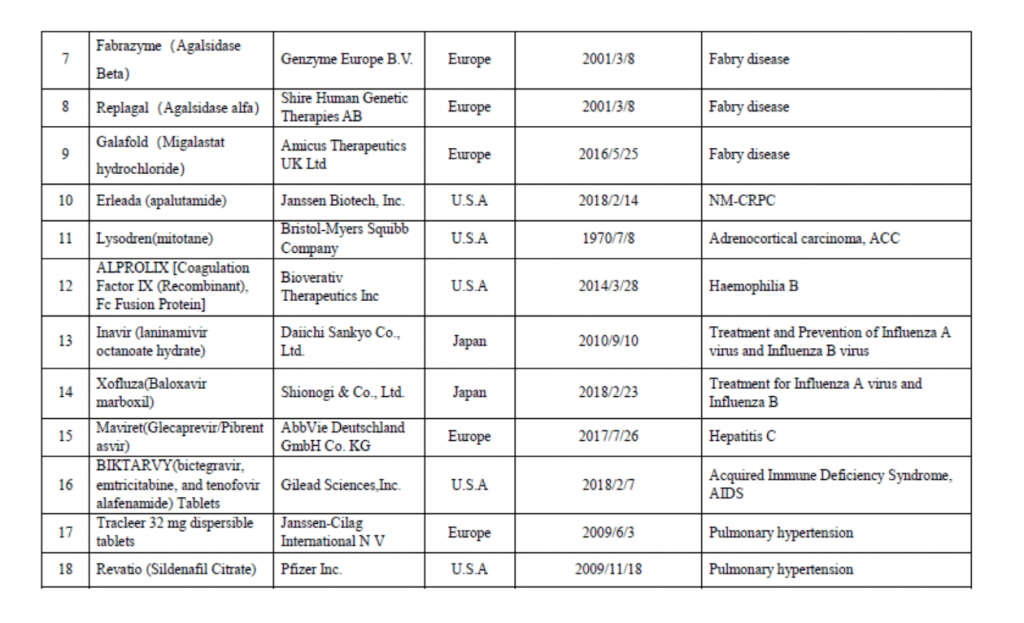
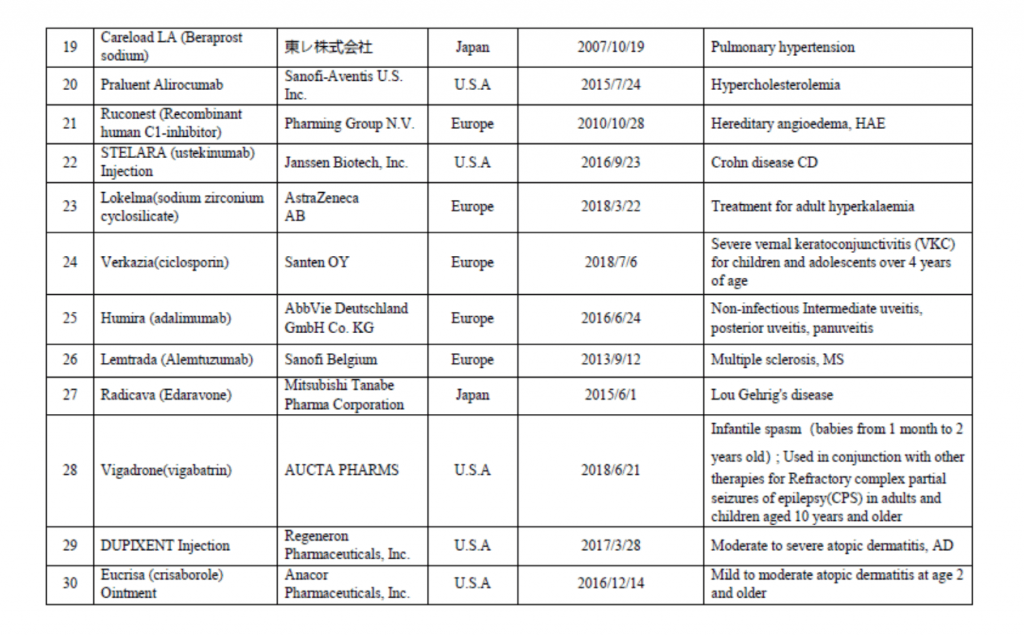
On March 28th, China Center for Drug Evaluation (CDE) released an “urgently needed” new drugs list for fast track, accelerated approval, priority review which contains a list of 30 overseas country approved new drugs. This list mainly refers to new drugs for the treatment of rare diseases and for the prevention and treatment of diseases that seriously endanger life or seriously affect quality of life, which have been existingly approved by US FDA, the European Union or Japanese drug authorities but have not been listed in China in recent years; drugs that are not yet available in China with no effective treatment or new drugs with obvious clinical significance. On August 8th year 2018, CDE published the first batch list of 48 new drugs that were approved overseas as the urgently needed in China.
Last year, Chinese state council executive meeting has proposed to fast track and grant accelerated approval of the urgently needed new drugs in China. In October 31th, 2018, National medical products administration (NMPA) issued an notice (No. 79), published “The review and approval process for urgent clinical needs foreign drug” which jointly work with National Health Commission of the People’s Republic of China. Priority review and a special evaluation channels will be established to fast track review and approve new drugs that are urgently needed. Those on the list and classified as rare disease drugs and other new drugs aims to gain China drug approval within 3 months and 6 months respectively. Please see the list below for your reference.
For more information please contact: info@accestra.com