What is Pharmacovigilance (PV)
- the monitoring,
- identification,
- evaluation,
- and control of adverse drug reactions (ADRs).
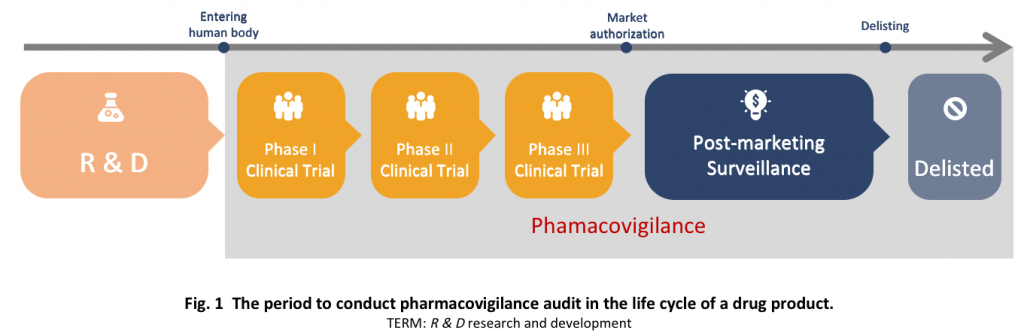
History of Pharmacovigilance (PV) in China
Stage One
Stage Two
Stage Three
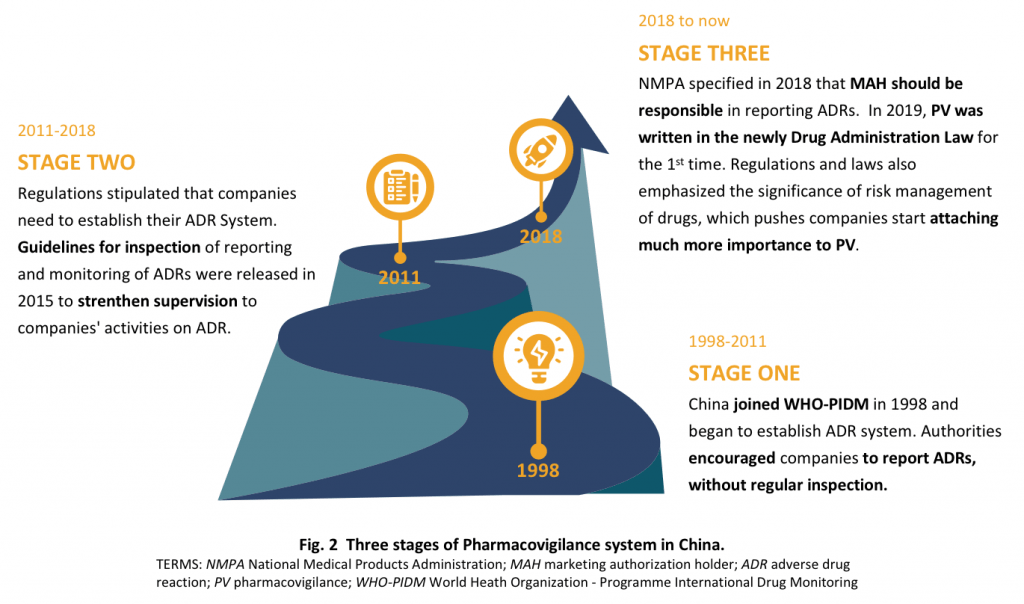
Important Regulatory Milestones
Ministry of Health
2011 – Measures for the reporting and monitoring of Adverse Drug Reaction.
ADR reporting system is established.
CFDA
2015 – Guidelines on inspection of the reporting and monitoring of adverse drug reactions (for trial implementation)
The monitoring, supervision, and inspection of ADR are strengthened.
State Council
2017 – Agenda of deepening reform of the examination and approval system and encouraging innovation of drugs and medical devices.
The system of MAH directly reporting ADRs is established.
NMPA
2018 – Announcement on Direct Reporting of Adverse Drug Reaction by Marketing Authorization Holder.
The responsibility of MAH in reporting ADRs is implemented.
Congress
2019 – Drug Administration Law.
PV is written into the basic law for the first time.
The main responsibilities of MAH in PV are emphasized.
NMPA
2020 – Good Pharmacovigilance Practices (draft for comments)
The main responsibilities of MAH in PV are standardized. Post-marketing management is strengthened.
Post-marketing Responsibilities of MAH in PV
1. Establishment of PV System
- Choose persons in charge of ADR monitoring.
- Establish specialized departments.
- Appoint specialists.
- Set up and improve relevant management systems.
2. Monitor and Report ADRs
- Establish channels for collecting ADR information.
- Actively collect suspected adverse reactions from drug use in a comprehensive manner.
- Report ADRs in time as required.
- Medical facilities,
- enterprises,
- phones,
- academic literature,
- post-marketing safety studies and projects,
- overseas information.
3. Identify and assess safety risks
- Identify potential risks of drugs and conduct studies on the mechanism and causes of those risks.
- Actively carry out post-marketing safety studies.
- Conduct ongoing risk evaluation and benefits of drugs.
- Prepare and submit annual reports and Periodic Safety Update Reports (PSUR).
4. Risk Control of Drug Safety
5. Create and maintain PV System Master Files (PSMF)
- Create and maintain PV system master files to describe the PV system and activities.
- Update the master files on time to ensure that they are consistent with the current PV system and activities, and conform to relevant laws and regulations, and practical work requirements
Services Accestra Consulting Group can provide
We provide services for Post-marketing Pharmacovigilance (PV) related activities in China, including:
- Establishment and Improvement of Post-marketing PV System.
- Processing of Post-marketing Individual Case Safety Reports (ICSRs).
- Medical Literature Searching.
- Periodic Safety Update Report (PSUR) Preparation.
- Safety Annual Report Preparation.
- PV System Master Files (PSMF) Preparation & Update on behalf of the client.