Since June 2017, China National Medical Products Administration (NMPA) has become a member of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), which indicates the conformity of China technical requirements for drugs and other pharmaceutical products with the international guidelines. Since then, major regulatory reforms are being made for drug administration, research and pharmaceutical industry in China. From February 1, 2018, application dossiers for clinical trial approval, chemical drug registration and therapeutic biological product registration are required to be submitted in Common Technical Document (CTD) format. In 2019, NMPA has released guidelines for eCTD submission, including specifications, validation criteria and submission requirements. A grand launch of China eCTD is just around the corner. It is time to get ready.
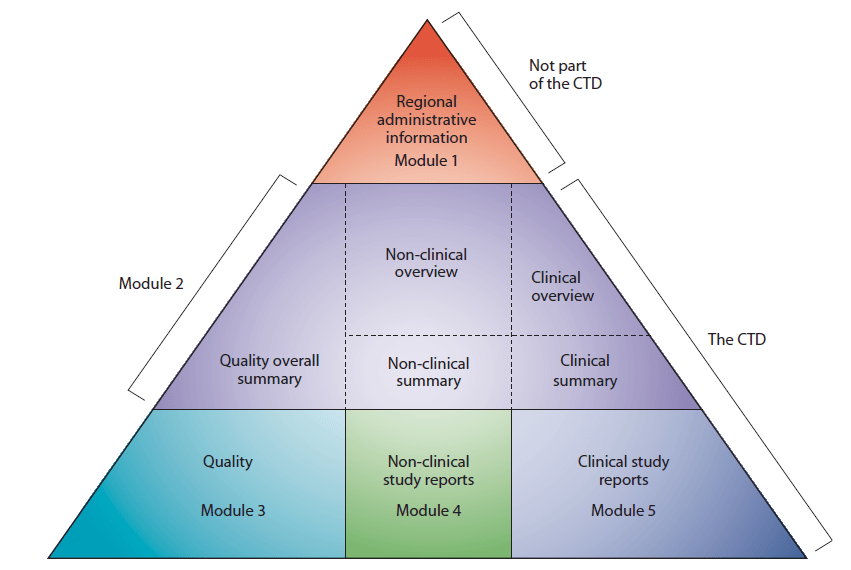
If you are unfamiliar with the CTD format of application dossier, please see CTD organization in the “CTD triangle” below (Fig. 1).