On May 9th of 2019, China National Medical Products Administration released its annual statistical report on drug regulation and inspection for the year 2018. This official annual report gave the statistics from five aspects, including production and business license, registration and approval, protection of traditional Chinese medicine varieties, complaint reporting and the investigation/ punishment cases.
Official link of the annual report: http://www.nmpa.gov.cn/WS04/CL2151/337665.html
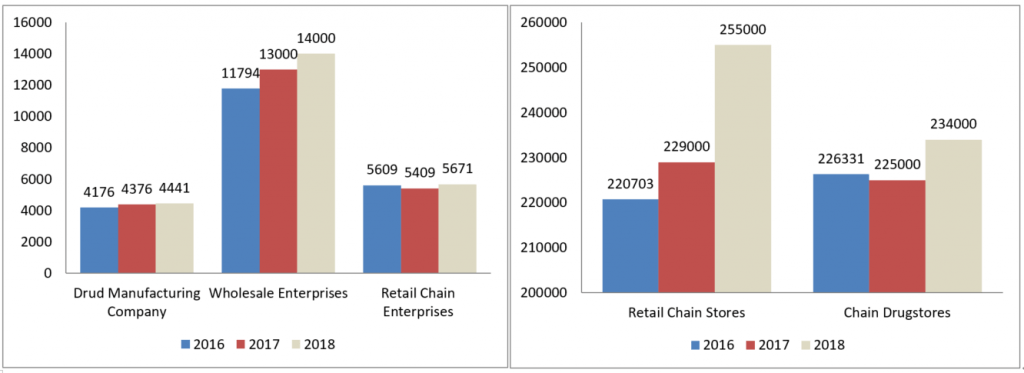
1.China’s drug manufacturing and operating license
It can be indicated from the statistical chart below there is steady increase of licensed API and drug product manufacturing company from year 2016 to year 2018, while the number of retail chains and drugstores are increasing significantly. Large pharmacy chain companies are gaining more market share through acquisition and expansion by opening more regional stores.
2. Drug registration application and approval statistic
- There are 312 new drug clinical trial applications approved by China CDE in year 2018;
- There are 58 generic drug clinical trial applications approved by China CDE while there are 464 total applications in year 2018;
- China CDE accepted a total of 154 applications for imported drugs, and total marketing approval number of imported drugs is 90.
- The state administration approved a total of 1862 supplementary drug applications. A total of 3,276 supplementary drug applications were approved and 12,648 were filed by the provincial (district, municipal) bureaus
3. Protection of Traditional Chinese Medicine Varieties
By the end of November 2018, there were 192 certificates of Traditional Chinese Medicine protected varieties, including 99 for the first time, 4 for the same variety and 89 for extended protection period.
4. Complaint reporting
In 2018, a total of 64,000 drug complaints and reports were accepted by regulators at all levels, with 3,556 cases registered and 4,036 cases closed.
5. Investigation/ punishment cases
In 2018, a total of 98,000 drug cases were investigated and punished by regulators at all levels, with 2.74 billion Yuan worth of goods, 7.66 billion Yuan fines, 2.02 billion Yuan illegal gains were confiscated. Also, there are 1,037 unlicensed businesses banned, 148 fake production and sales dens destroyed, 1,093 enterprises were ordered to suspend production and business, 197 licenses revoked and 2,000 licenses transferred to judicial organs.
In 2018, regulators at all levels investigated and dealt with 249 cases of pharmaceutical packaging materials, with total value of 1.264 million yuan worth of goods.








