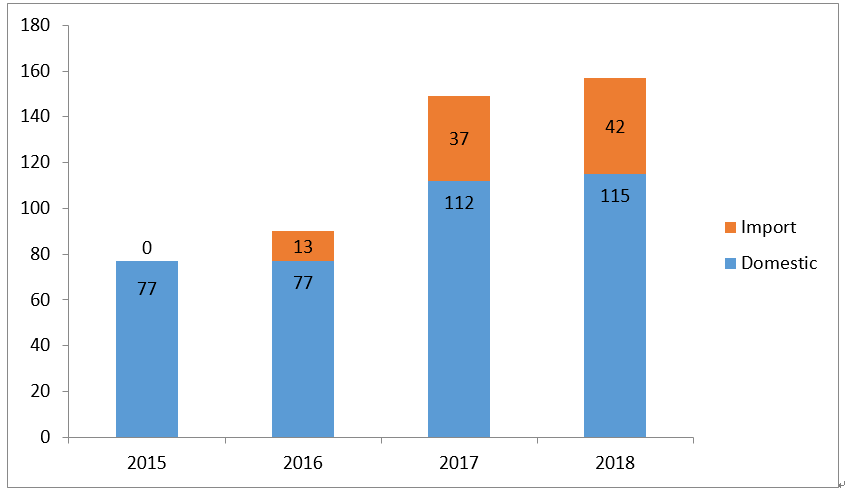On 2nd July, 2019, China CDE-NMPA published the 2018 working Report, summarizing the drug review and approval statistic data in the past year.
See below for the details.
1- Overall Statistic
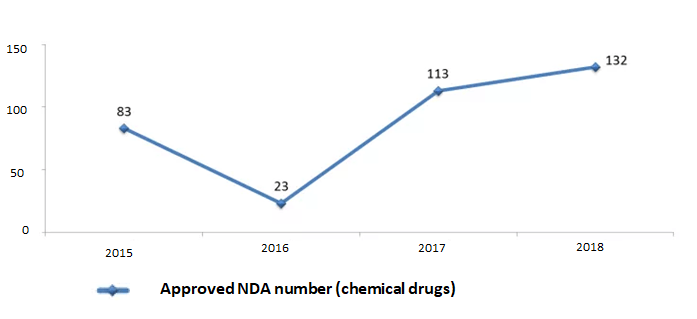
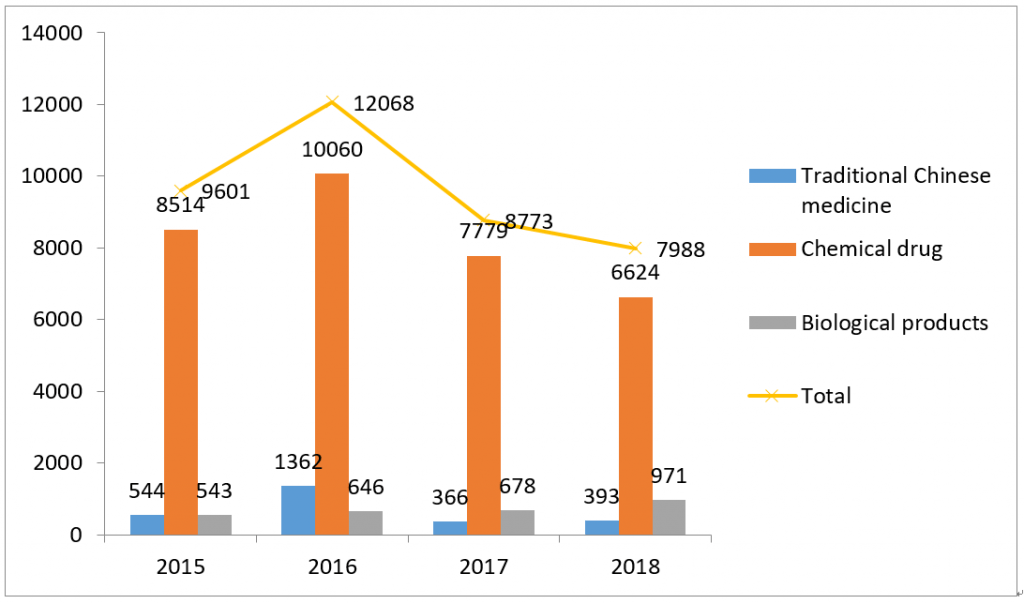
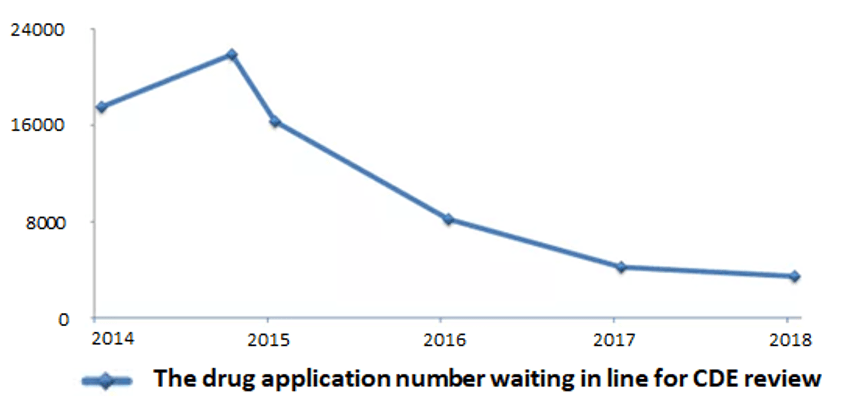
It can be seen from Figure 1, 2, 3 and 4, the backlog of drug applications in CDE has been greatly alleviated: the application number waiting in line has dropped from nearly 22,000 at the peak of year 2015 to 3,440 in year 2018; the review completion rate for all types of drug applications in 2018 has exceeded 90%.
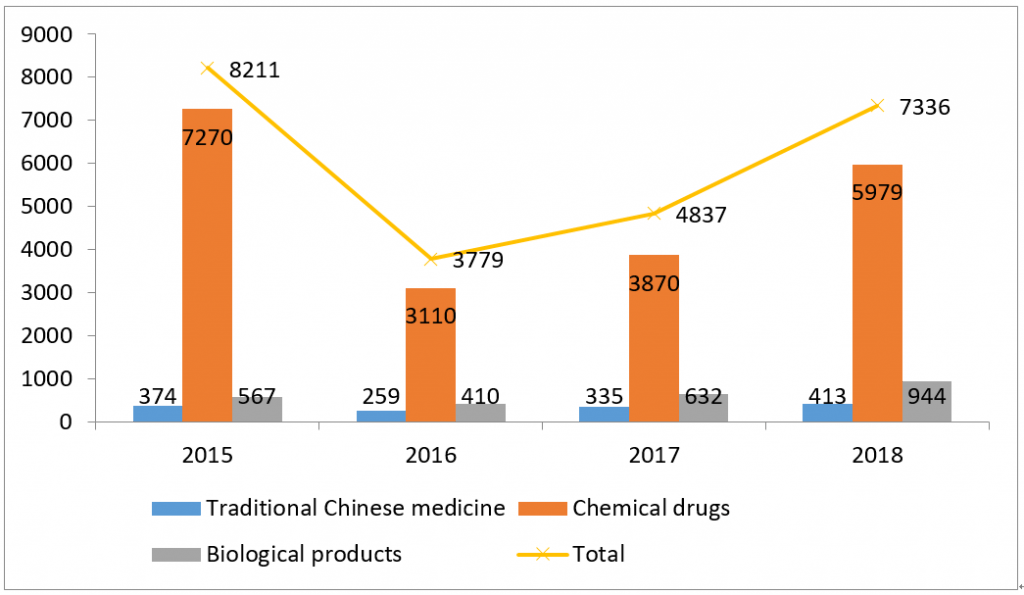
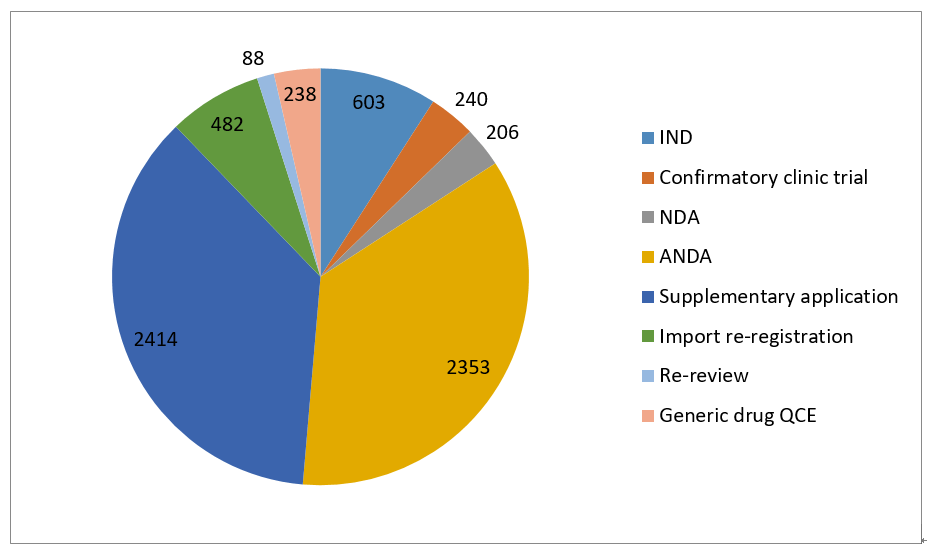
(By different drug category)
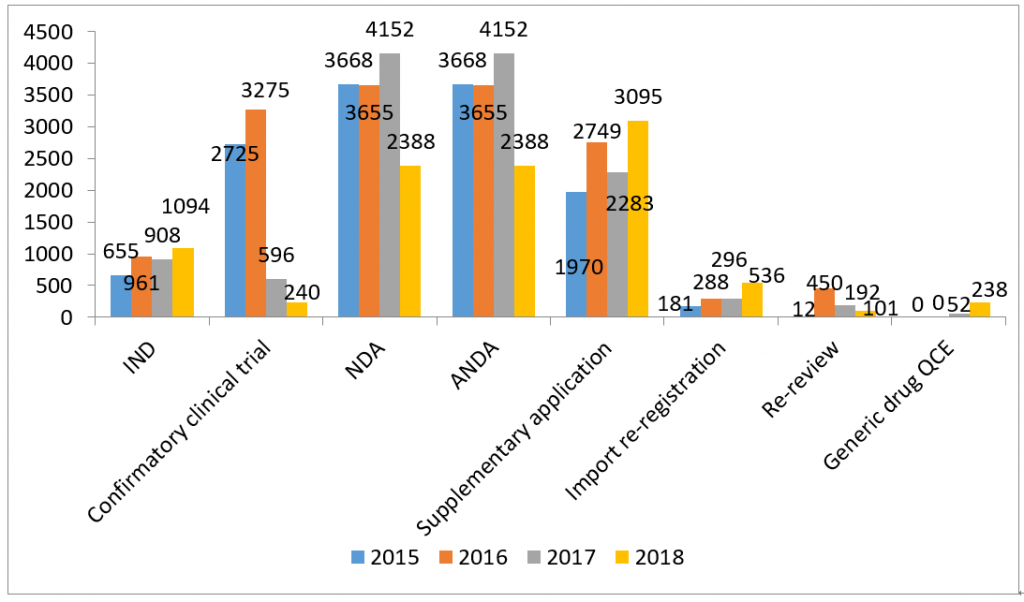
(By different application type)
2- Statistic of Chemical Drugs -Overall
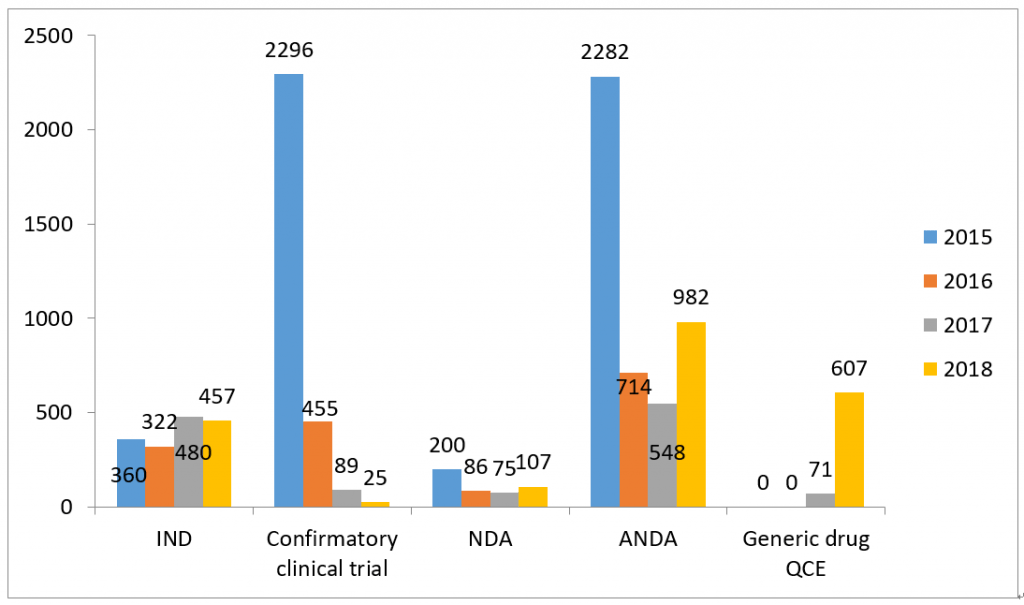
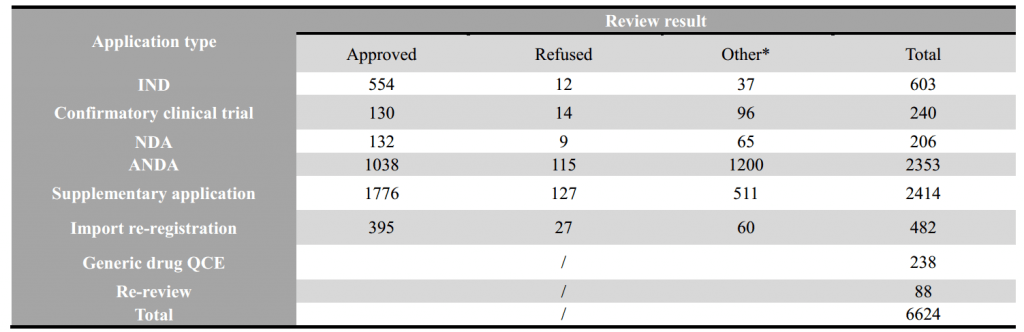
*Note: “other” refers: 1) the applicant withdrawal the application, 2) Supplementary information is required from applicant after the review, 3) the application directly submitted to Drug Registration Management Bureau of NMPA instead of CDE, 4) the combination application of medical device, 5) API/excipient/package material application for linked review procedure.
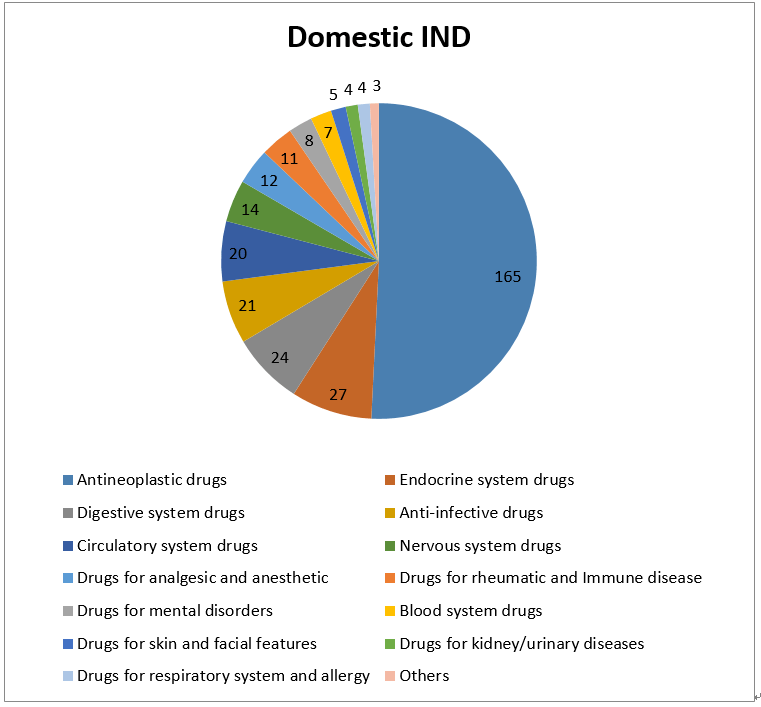
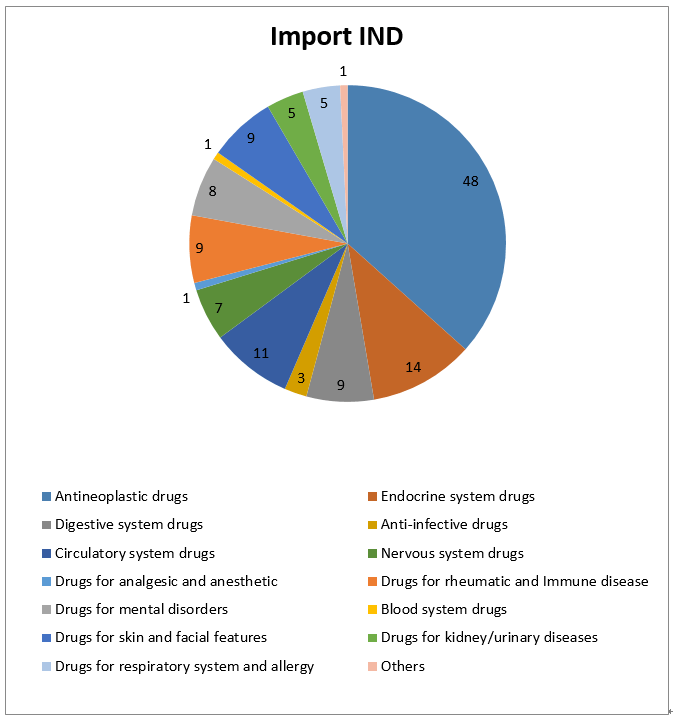
3- Statistic of Chemical Drugs -IND
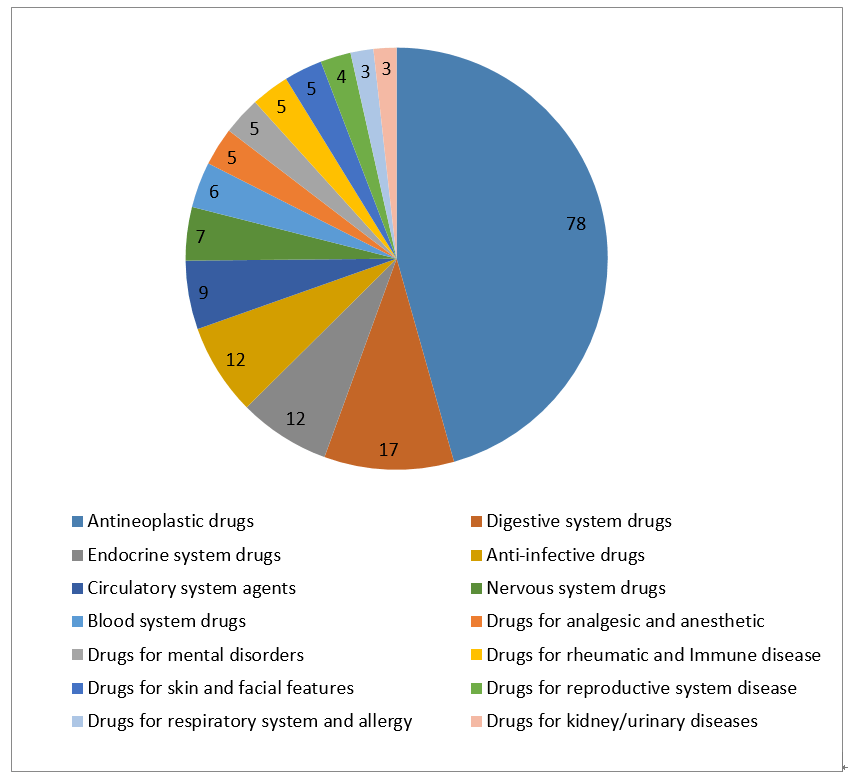
4- Statistic of Chemical Drugs -NDA