Table of contents1. Summary. |
In 2020, the Center for Drug Evaluation (CDE) of China reviewed and/or approved 11,582 drug registrations. To summarize the accomplishments and failures of drug approvals, CDE released a China Drug Evaluation Report of the year. Part 1 of the report gives an overview of drug approvals for:
|
1. China Drug Evaluation Report Summary
|
CATEGORY |
REGISTRATION TYPE |
NUMBER OF REGISTRATIONS |
|---|---|---|
| Drug Product | Requires Technical Review | 8,606 |
| Only Administrative Approval | 2,972 | |
| Drug-device Combination | 4 | |
| Total Number | 11,582 |
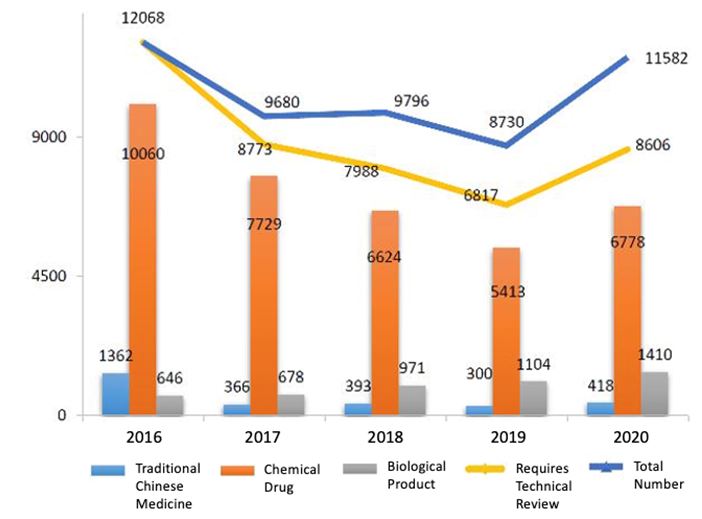
- 6,778 chemical drugs (25.33% increase year on year)
- 418 traditional Chinese medicines (39.33% increase year on year),
- 1,410 biological products (27.72% year on year).
View the diagram below (Fig. 1) for a comparison between the categories and the total number of registrations reviewed between 2016 and 2020.
Fig. 1 Comparison of Drug Registrations Reviewed from 2016 to 2020.
(Note: There was no registration that required only administrative approval in 2016, i.e., all registrations required technical review & approval. From 2017, specific registrations could be approved without technical review under the new regulatory framework.)
2. Technical Review & Approval
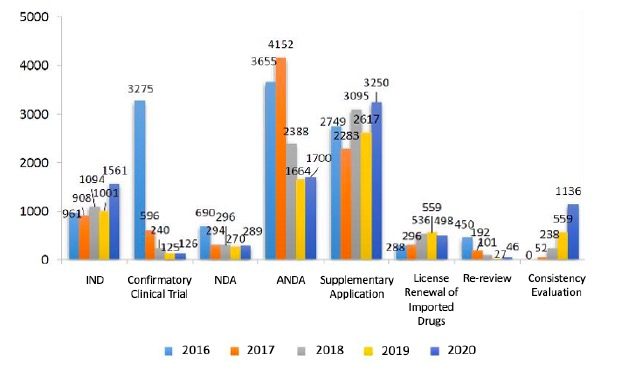
- 1,561 Investigational New Drug (IND) applications (55.94% increase year on year ),
- 289 New Drug Applications (7.04% increase year on year ),
- 1,700 Abbreviated New Drug Applications (2.16% increase year on year).
Fig. 2 Number of Applications Reviewed from 2016 to 2020. (Note: CDE has started to conduct quality and efficacy consistency evaluation of generic drugs since August 2017.)
Table 2. Overview of New Drug Approvals for Hot Topics in 2020.
|
NO. |
TOPIC IN DRUG THERAPY |
INDICATION |
DRUG CATEGORY |
|---|---|---|---|
| 1 | COVID-19 Vaccines and other treatments | SARS-CoV-2 | COVID-19 Vaccine (Vero Cell) Inactivated |
| 2 | COVID-19 Vaccines and other treatments | Acute Lung Injury (ALI) / Acute Respiratory Distress Syndrome (ARDS) | Liahnhua Qingwen Tables/Capsules/Jinhua Qinggan Tables / Xuebijing Injection |
| 3 | COVID-19 Vaccines and other treatments | Non-small Cell Lung Cancer | Sivelestat Sodium Hydrate for Ijection |
| 4 | Anti-neoplastic Drugs | Non-small Cell Lung Cancer | Almonertinib Mesylate Tablets |
| 5 | Anti-neoplastic Drugs | Pancreatic and Non-pancreatic Neuroendocrine Tumors | Surufatinib Capsules |
| 6 | Anti-neoplastic Drugs | Hodgkin Lymphoma and Anaplastic Large Cell Lymphoma | Bretunximab Vedotin for Injection |
| 7 | Anti-neoplastic Drugs | B-precursor Acute Lymphoblastic Leukaemia | Blinatumomab for Injection |
| 8 | Anti-neoplastic Drugs | Differentiate Thyroid Cancer | Lenvatinib Mesylate Capsules |
| 9 | Anti-infective Drugs | Chronic Hepatitis C | Coblopasvir Hydrochloride Capsules |
| 10 | Anti-infective Drugs | HIV-1 Pre-Exposure Prophylaxis | Emtricitabine / Tenofovir Disoproxil Fumarate Tablets |
| 11 | Circulatory System Drugs | Hereditary Angioedema | Lanadelumab-flyo Injection |
| 12 | Circulatory System Drugs | Transthyretin Amyloidosis | Tafamidis Soft Capsules |
| 13 | Respiratory System Drugs | Persistent Allergic Rhinitis | Bencycloquidium Bromide Nasal Spray |
| 14 | Respiratory System Drugs | Idiopathic Pulmonary Fibrosis (IPF)/Systemic Sclerosis Associated Interstitial Lung Disease (SSc-ILD) | Nintedanib Esylate Soft Capsules |
| 15 | Nervous System Drugs | Huntington’s Disease | Deutetrabenazine Tablets |
| 16 | Nervous System Drugs | Stage 1 Symptomatic Polyneuropathy | Tafamidis Meglumine Soft Capsules |
| 17 | Analgesics and Anesthetics | Painless Gastroenteroscopy | Ciprofol Injection |
| 18 | Dermatological and Otorhinolaryngological Drugs | Neurotrophic Keratitis | Cenegermin Eye Drops |
| 19 | Dermatological and Otorhinolaryngological Drugs | Atopic Dermatitis | Dupilumab Injection |
| 20 | Gastrointestinal Drug | Ulcerative Colitis/Crohn’s Disease | Vedolizumab for Injection |
| 21 | Surgical Drugs | Malignant Hyperthermia | Tacrolimus Granules |
| 22 | Surgical Drugs | Pediatric Transplant Rejection | Tacrolimus Granules |
| 23 | Rare Disease Drugs | Mucopolysaccharidosis 1 | Laronidase Concentrate for Injection |
| 24 | Rare Disease Drugs | Mucopolysaccharidosis 2 | Idursulfase-beta Injection |
| 25 | In Vivo Diagnostics | In-vivo diagnosis of mycobaterium tuberculosis infection | Recombinant ESAT-6:CFP-10 Fusion Protein |
| 26 | Preventive Biological Product (Vaccine) | Influenza | Influenza Vaccine, Live, Nasal, Freeze-dried |
| 27 | Traditional Chinese Medicine | Type 2 Diabetes | Mulberry Twig Alkaloids Tablets |
| 28 | Traditional Chinese Medicine | Knee Osteoarthritis | Jingu Zhitong Gel |
| 29 | Traditional Chinese Medicine | Acute Bronchitis and Tracheitis | Lianhua Qingke Tablets |
3. Reasons for Application Rejections
- Insufficient proof for drug safety,
- Efficacy,
- Quality control or delay in submitting the supplementary materials upon CDE’s requests.
3.1 New Drug Application Rejections
3.1.1. IND Application Rejections.
-
Lack of pre-IND meeting
Major data gaps were identified after IND applications were filed and couldn’t be supplemented in the given timeline.
-
Insufficient data for clinical development justification.
a. The IND-enabling (incl. pharmaceutical, nonclinical, preclinical) studies demonstrated that the drug efficacy was low and safety risk was high. The risk-benefit ratio was not suitable for first-in-human testing.
b. The clinical development purpose did not comply with the principles of clinical trials.
c. The IND-enabling studies were insufficient to start clinical trials.
-
Insufficient data to enable clinical studies or control safety risks in humans.
a. Major defects in the clinical trial protocol and insufficient risk control measures.
b. Insufficient nonclinical data for combination therapy drugs.
c. Insufficient data for each individual vaccine or different immunization procedures between the individual vaccines of a combination vaccine.
3.1.2. NDA Rejections
-
Major defects in the research quality control and management system. Thus the submitted data couldn’t prove the safety, efficacy, and quality of the drug.
-
Lack or regulatory compliance
a. Inconsistent study drugs were used for different phases of clinical trials.
b. Authenticity issues were identified in clinical data during on-site inspection/audit.
3.2. ANDA Rejections
-
Unsuitable justification for generic development: The reference listed drug (RLD) which the generic compared to has been withdrawn from the market. A new drug with improved safety has been authorized for marketing in China.
-
Insufficient data for quality consistency
a. Product specification review and sample inspection did not follow the requirements, or major defects were identified in the analytical methods.
b. The bioequivalence study demonstrated that the generic and RLD were not bioequivalent.
c. Lack of compliance with technical requirements for generics (such as stability testing and choose of API starting materials).
d. The APIs were not from legal sources.
3.3. Supplementary Application Rejections
-
Insufficient data to justify the change to an approved drug.
a. The submitted change had a major impact on the drug substance and caused substance change.
b. The submitted change to the drug label/insert did not follow technical requirements for drafting the drug label/insert.
-
The submitted data could not prove that the change to an approved drug had no impact on the drug safety, efficacy, and quality (such as insufficient reference literature and clinical data).
3.4. Other Application Rejection
-
Biosimilars.
a. Lack of similarity assessment or the choice of reference drug in the comparative study did not follow requirements.
b. Insufficient nonclinical/preclinical data to enable clinical studies.
-
Natural Medicines: The submitted data did not follow requirements for multi-regional clinical trials or the national guidelines for the review of natural medicines.
- Drugs/Pharmaceutical,
- Food and Feed products for China NMPA (Formerly CFDA).
- Regulatory approval including product registration for China Investigational New Drug (IND),
- China New Drug Application (NDA),
- China Drug Master File (DMF) for APIs,
- Excipients and Packaging Materials,
- ANDA,
- eCTD submission and others.