Part 2. Chemical Drugs, Biological Products and Traditional Chinese Medicines
Table of contents1. Chemical Drugs. |
Despite severe challenges from the COVID-19 pandemic, the Center for Drug Evaluation (CDE) of China reviewed 8,606 drug applications in 2020 and approved many new drugs to help patients suffering from different diseases.
As a follow-up on part 1 of the Drug Evaluation Report 2020, part 2 gives an overview of drug applications and approvals respectively for chemical drugs, biological products, and traditional Chinese medicines. |
1. Chemical Drugs
1.1 Application Overview
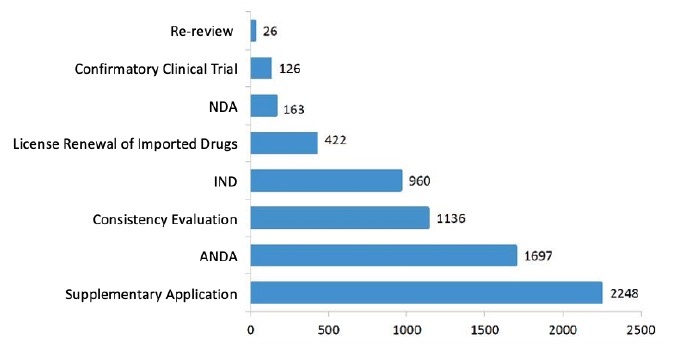
- 1,086 Clinical Trial Applications (CTAs) for Investigational New Drugs (INDs) and confirmatory trials,
- 163 New Drug Applications (NDAs),
- 1,697 Abbreviated New Drug Applications (ANDA),
- 1,136 consistency evaluations,
- 2,248 supplementary applications.
1.2. Approval
|
APPLICATION TYPE |
NUMBER OF APPLICATIONS |
|||
|---|---|---|---|---|
|
APPROVAL (INCL. AFTER MATERIAL SUPPLEMENT) |
NO APPROVAL SUGGESTED/NOT APPROVED |
OTHERS* |
TOTAL NUMBER |
|
| IND | 907 | 39 | 14 | 960 |
| Confirmatory Clinical Trial | 108 | 11 | 7 | 126 |
| NDA | 115 | 3 | 45 | 163 |
| ANDA | 918 | 32 | 747 | 1,697 |
| Supplementary Application | 1,732 | 126 | 390 | 2,248 |
| License Renewal of Imported Drugs | 380 | 17 | 25 | 422 |
| Consistency Evaluation | 577 | 12 | 547 | 1,136 |
| Re-Review | N/A | 26 | ||
| Total | 4,737 | 240 | 1,775 | 6,778 |
Others*: herein refers to the applications which were withdrawn by the applicants or pending for material supplement after technical review, similarly hereinafter.
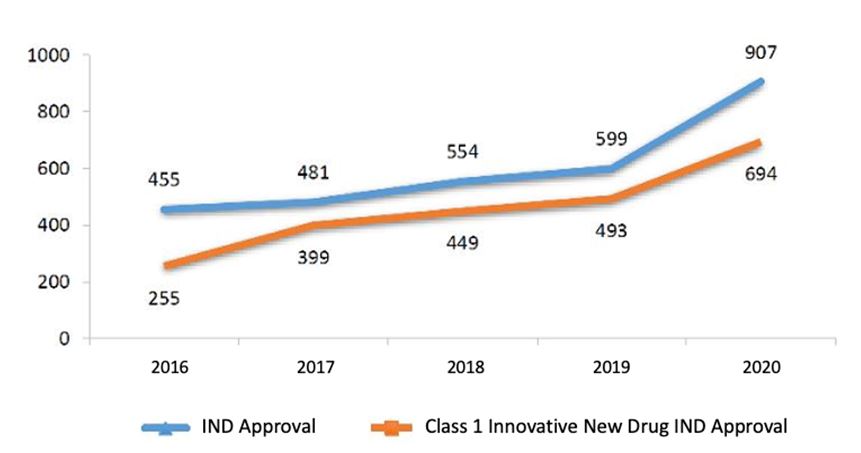
1.2.1. IND Approval
- Anti-neoplastic,
- anti-infective,
- circulatory system,
- endocrine system,
- gastrointestinal,
- autoimmune disease,
- immunosuppressant drugs.
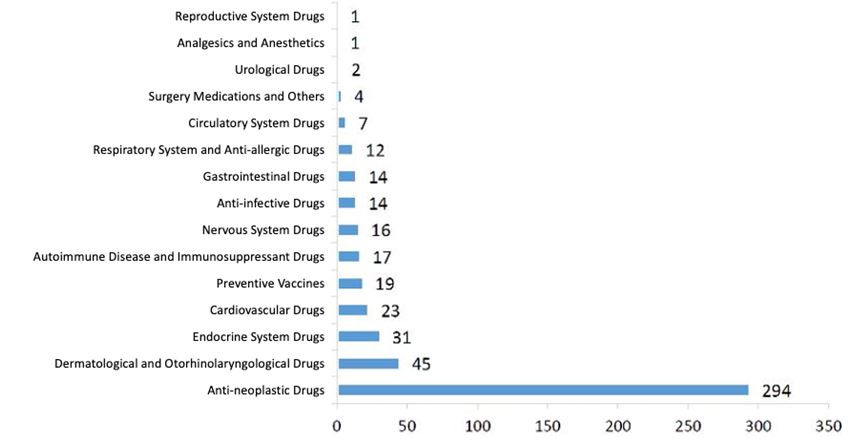
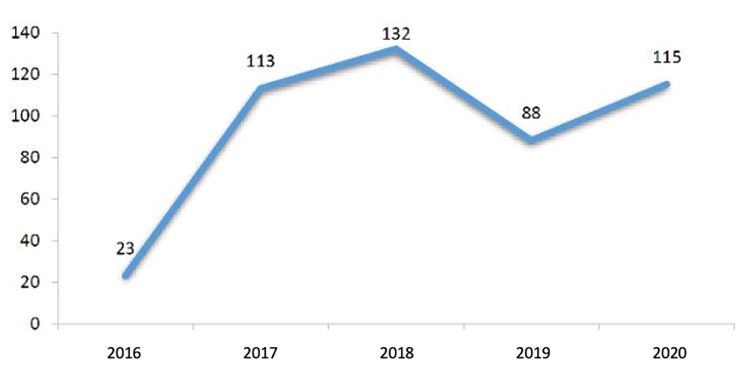
1.2.2. NDA Approval
2. Biological Products
2.1 Application Overview
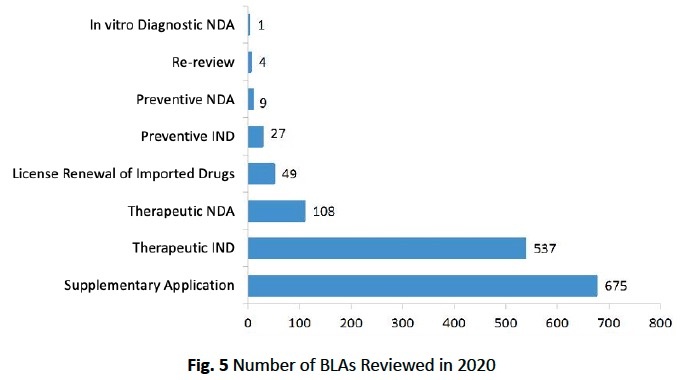
The number of reviewed IND applications and NDAs is given in the below table (Table 2) respectively for preventive and therapeutic biological products and in vitro diagnostics.
Table 2. Number of INDs and NDAs Reviewed for Biological Products in 2020
|
CLASSIFICATION |
NUMBER OF INDs |
NUMBER OF NDAs |
|---|---|---|
| Preventive Biological Product | 27 | 9 |
| Therapeutic Biological Product | 537 | 108 |
| In-vitro Diagnostics | 0 | 1 |
The diagram below (Fig. 5) shows the number of BLAs reviewed for every application type.
2.2 Approval
The number of approvals for each application type is summarized in the table below (Table 3).
Table 3. Overview of BLA Approvals in 2020
|
APPLICATION TYPE |
NUMBER OF APPLICATIONS |
|||
|---|---|---|---|---|
|
|
APPROVAL (INCL. AFTER MATERIAL SUPPLEMENT) |
NO APPROVAL SUGGESTED/NOT APPROVED |
OTHERS* |
TOTAL NUMBER |
| Preventive IND | 19 | 4 | 4 | 27 |
| Therapeutic IND | 481 | 45 | 11 | 537 |
| Preventive NDA | 7 | 0 | 2 | 9 |
| Therapeutic NDA | 81 | 1 | 26 | 108 |
| In vitro Diagnostic NDA | 1 | 0 | 0 | 1 |
| Supplementary Application | 551 | 22 | 102 | 675 |
| License Renewal of Imported Drugs | 45 | 0 | 4 | 49 |
| Re-Review | N/A | N/A | N/A | 4 |
| In Total | 1,185 | 72 | 149 | 1,410 |
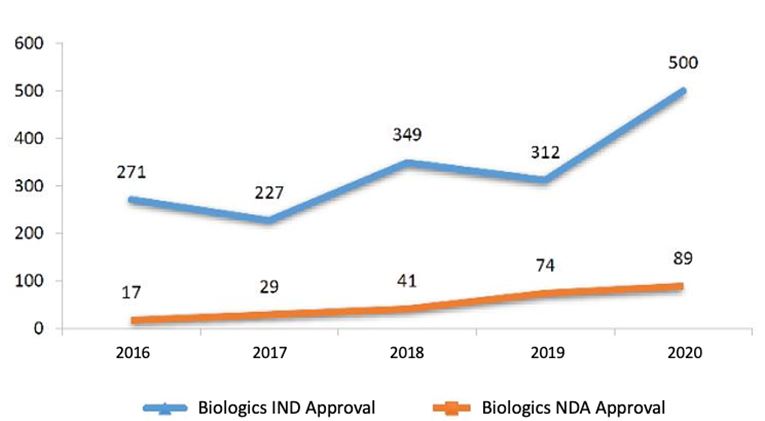
2.2.1. IND Approval

2.2.2. NDA Approval
3. Traditional Chinese Medicines
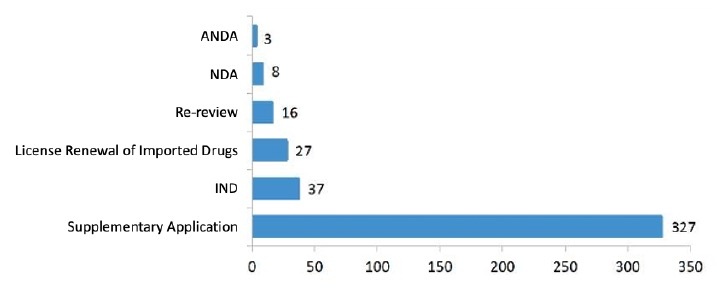
3.1 Application Overview
3.2 Approval
Among the reviewed TCM applications, 28 INDs and 4 NDAs were approved by CDE. The number of approvals for each application type is summarized in the table below (Table 4).
Table 4. Overview of TCM Approvals in 2020
|
APPLICATION TYPE |
NUMBER OF APPLICATIONS |
|||
|---|---|---|---|---|
|
Approval (incl. approval after material supplement) |
No Approval Suggested / Not Approved |
Others* |
Total Number |
|
| IND | 28 | 5 | 4 | 37 |
| NDA | 4 | 0 | 4 | 8 |
| ANDA | 0 | 2 | 1 | 3 |
| Supplementary Application | 220 | 42 | 65 | 327 |
| License Renewal of Imported Drugs | 17 | 6 | 4 | 27 |
| Re-Review | N/A | 16 | ||
| In Total | 269 | 55 | 78 | 418 |
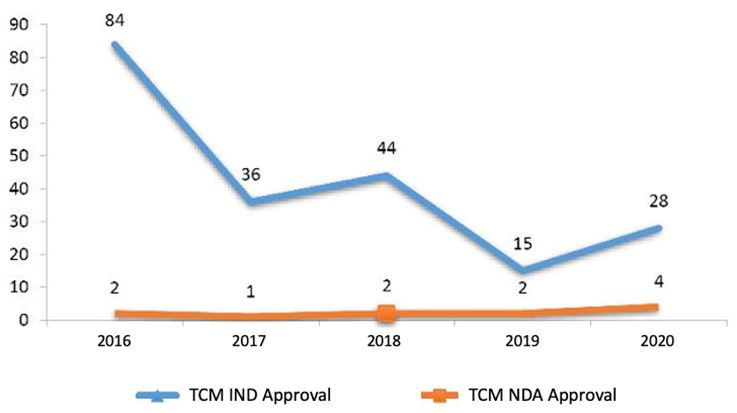
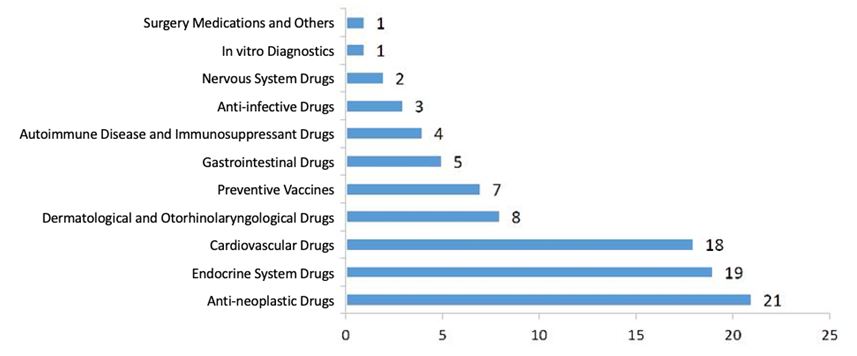
3.2.1. IND Approval
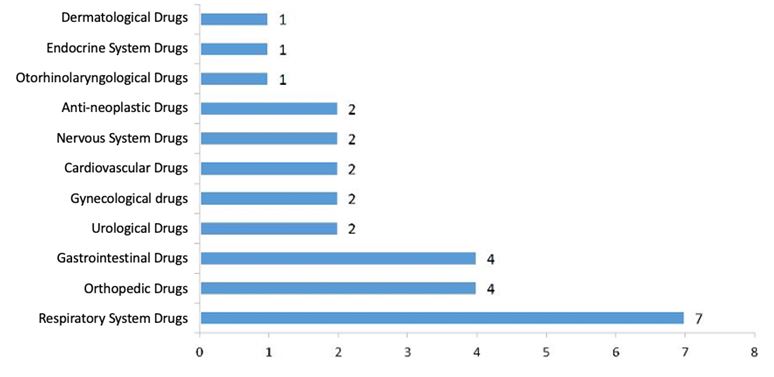
- Drugs/Pharmaceutical,
- Food and Feed products for China NMPA (Formerly CFDA).
- Regulatory approval including product registration for China Investigational New Drug (IND),
- China New Drug Application (NDA),
- China Drug Master File (DMF) for APIs,
- Excipients and Packaging Materials,
- ANDA,
- eCTD submission and others.