In 2019, the Center for Drug Evaluation of China reviewed and approved a total of 8730 drug registration applications. As a follow-up on part 1 of Drug Evaluation Report 2019, part 2 gives an overview of technical review and approval of these registration applications, and an analysis of those approved under each registration classification, including chemical drugs, traditional Chinese medicines and biological products.
1. Overall Summary
1.1 Executive Summary
| APPROVAL TYPE | NUMBER OF APPLICATIONS | |
|---|---|---|
| DRUG PRODUCTS | 4075 | |
| Technical Review & Approval | National Review & Approval (by CDE) | 2742 |
| Provincial Review & National Approval | ||
| ADMINISTRATIVE APPROVAL | 1908 | |
| Drug-device combinations | 5 | |
| Total Number | 8730 |
Table 1. Number of applications reviewed and approved in 2019.
For applications under or pending for review and approval, the number of applications under each status is listed in Table 2. For applications with completed technical review, the number of applications under each drug classification is listed in Table 3.
| STATUS OF REVIEW | NUMBER OF APPLICATIONS | |
|---|---|---|
| Initiation | 3334 | |
| Pending for | On-site inspection | 450 |
| Product dossier of API, Excipient, Packaging | 290 | |
| Validation of process, specifications, package, inserts and labels | 235 | |
| Sample Testing | 36 | |
| TOTAL NUMBER | 4423 |
Table 2. Number of applications under or pending review and approval in 2019.
| DRUG CLASSIFICATION | NUMBER OF APPLICATIONS | % OF TOTAL APPLICATIONS |
|---|---|---|
| Chemical Drug | 5413 | 79.40% |
| Biological Product | 1104 | 16.20% |
| Traditional Chinese Medicine | 300 | 4.40% |
| TOTAL NUMBER | 6817 | 100.00% |
Table 3. Number of applications with completed technical review of 2019.
1.2 Overview of Completed Technical Review
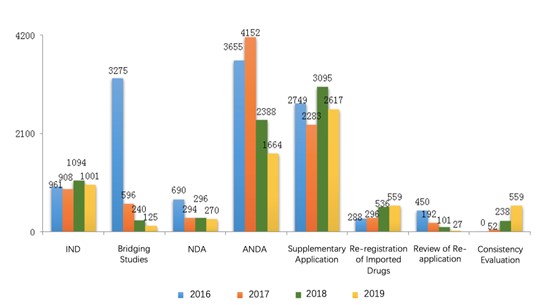
In 2019, CDE completed the technical review for 6817 drug registration applications, among which the number of applications of each application type is shown in the diagram below (Fig. 1) along with the data from 2016 to 2018.
1.3 Overview of Approval
The table below (Table 4) shows the number of applications approved under each registration classification of chemical drugs, including Investigational New Drug (IND), New Drug Application (NDA) and Abbreviated New Drug Application (ANDA). As highlights, 493 applications for Class 1 Innovative New Drug were approved under IND registration while 260 applications for oral solid dosage forms were approved under ANDA.
| REGISTRATION CLASSIFICATION | NUMBER OF APPLICATIONS |
|---|---|
| IND | 926 |
| Class 1 Innovative New Drugs | 493 |
| Others | 433 |
| NDA | 164 |
| ANDA | 654 |
| Oral Solid Dosage Forms | 260 |
| Others | 394 |
| TOTAL NUMBER | 1744 |
Table 4. Number of applications approved under each chemical drug registration classification.
2. Analysis of Registration Applications
2.1 Chemical Drug Registration Applications
2.1.1 Overview
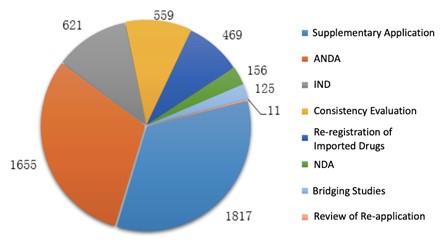
In 2019, CDE completed the technical review for 5413 chemical drug registration applications, among which the number of applications of each application type is shown in the diagram below (Fig. 2).
2.1.2 Approval
Among the chemical drug registration applications, 3412 applications were approved for technical review. For each application type, the number of approvals is listed in Table 5.
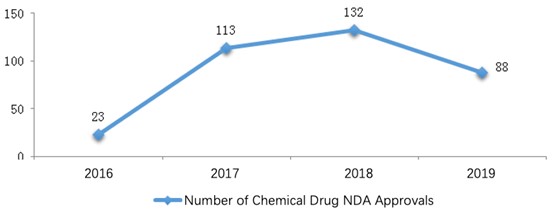
Under the registration classification New Drug Application (NDA), 88 applications were approved in 2019.
For a comparison with the last three years, the numbers of approvals from 2016 to 2019 are shown in the diagram below (Fig. 3).
| APPLICATION TYPE | APPROVAL (INCL. APPROVAL AFTER MATERIAL SUPPLEMENT) | NO APPROVAL SUGGESTED / NOT APPROVED | OTHERS* | TOTAL NUMBER |
|---|---|---|---|---|
| IND | 599 | 18 | 4 | 621 |
| Bridging Studies | 104 | 7 | 14 | 125 |
| NDA | 88 | 3 | 65 | 156 |
| ANDA | 654 | 71 | 930 | 1655 |
| Supplementary Application | 1309 | 85 | 423 | 1817 |
| Re-registration of imported drugs | 387 | 6 | 76 | 469 |
| Consistency evaluation | 260 | 17 | 282 | 559 |
| Review of re-application | 11 | – | – | 11 |
| IN TOTAL | 34112 | 207 | 1794 | 5413 |
Table 5. Overview of approvals for Chemical Drug Registrations in 2019.
- The applications withdrawn by the applicants,
- Pending for material supplement after technical review,
- Submitted to NMPA’s Department of Drug Registration instead of CDE,
- For drug-device combination registration with NMPA’s Center for Medical Device Evaluation,
- Withdrawn from the technical review of drug products combined with their related APIs/excipients.
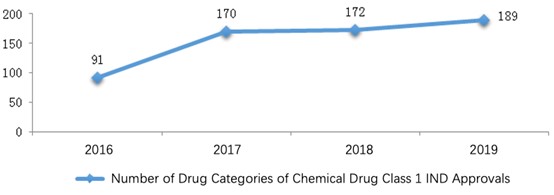
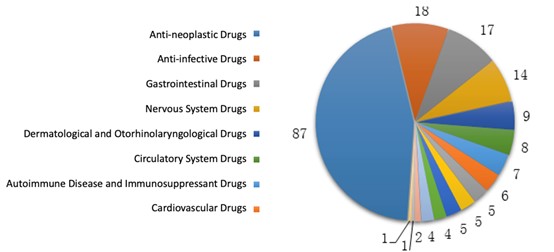
Among the 189 drug categories, anti-neoplastic, gastrointestinal, anti-infective and nervous system drugs covered 70% of the approvals. The diagram below (Fig. 5) shows the number of drug categories for each indication where Class 1 IND applications were approved in 2019.
2.2 Traditional Chinese Medicine Registration Applications
2.2.1 Overview
In 2019, CDE completed the technical review for 300 traditional Chinese medicine registration applications, among which the number of applications of each application type is shown in the diagram below (Fig. 6).
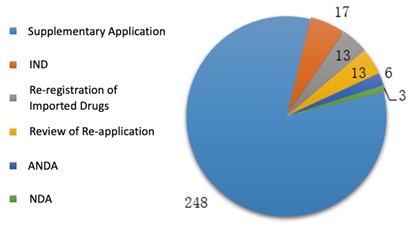
2.2.2. Approval
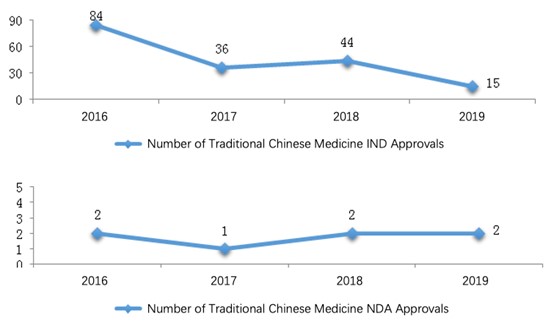
Among these registration applications, 15 IND applications and 2 NDAs for traditional Chinese medicines were approved for technical review.
For each application type, the number of approvals is listed in Table 6.
For a comparison with the last three years, the numbers of IND/NDA approvals from 2016 to 2019 are shown in the diagram below (Fig. 7).
| APPLICATION TYPE | NUMBER OF APPLICATIONS | |||
|---|---|---|---|---|
| APPROVAL (INCLU. APPROVAL AFTER MATERIAL SUPPLEMENT) | NO APPROVAL SUGGESTED / NOT APPROVED | OTHERS | TOTAL NUMBER | |
| IND | 15 | 2 | 0 | 17 |
| NDA | 2 | 0 | 1 | 3 |
| ANNDA | 0 | 5 | 1 | 6 |
| Supplementary Application | 195 | 2 | 51 | 248 |
| Re-registration of imported drugs | 6 | 0 | 7 | 13 |
| Review of re-application | 13 | 13 | ||
| IN TOTAL | 231 | 9 | 60 | 300 |
Table 6. Overview of approval for Traditional Chinese Medicine registrations in 2019.
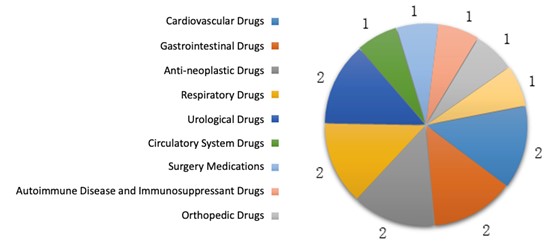
The approved traditional Chinese medicine IND registrations covered 10 indications, among which 10 approvals were for:
- cardiovascular,
- gastrointestinal,
- anti-neoplastic,
- respiratory,
- urological drugs (67% of the total number).
The diagram below (Fig. 8) shows the number of approvals for each indication of traditional Chinese medicine IND registrations in 2019.
2.3 Biological Product Registration Applications
2.3.1. Overview
In 2019, CDE completed the technical review for 1104 biological product registration applications, among which the number of applications of each application type is shown in the diagram below (Fig. 9).
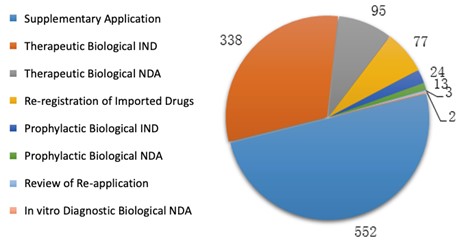
2.3.2 Approval
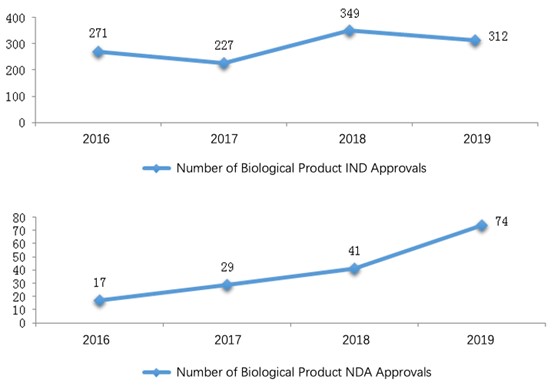
Among these registration applications, 312 IND applications and 74 NDAs for biological products were approved for technical review. For each application type, the number of approvals is listed in Table 7. For a comparison with the last three years, the numbers of IND/NDA approvals from 2016 to 2019 are shown in the diagram below (Fig. 10).
| APPLICATION TYPE | NUMBER OF APPLICATIONS | |||
|---|---|---|---|---|
| APPROVAL (INCL.APPROVAL AFTER MATERIAL SUPPLEMENT) | NO APPROVAL SUGGESTED / NOT APPROVED | OTHERS | TOTAL NUMBER | |
| Prophylactic Biological IND | 18 | 3 | 3 | 24 |
| Therapeutic Bilogical IND | 294 | 31 | 13 | 338 |
| Prophylactic Biological NDA | 5 | 1 | 7 | 13 |
| Therapeutic Bilogical NDA | 67 | 2 | 26 | 95 |
| In vitro Diagnostic Biological NDA | 2 | 0 | 0 | 2 |
| Supplementary Application | 361 | 14 | 177 | 552 |
| Re-registration of imported drugs | 62 | 1 | 14 | 77 |
| Review of re-applicaiton | 3 | 3 | ||
| IN TOTAL | 812 | 52 | 240 | 1104 |
Table 7. Overview of approvals for Biological Product REgistrations in 2019.
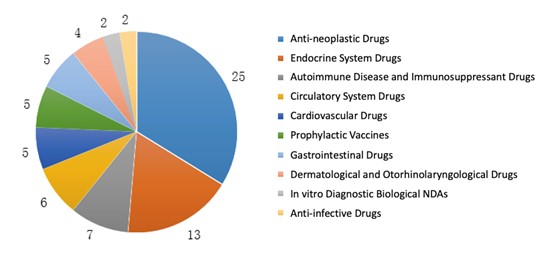
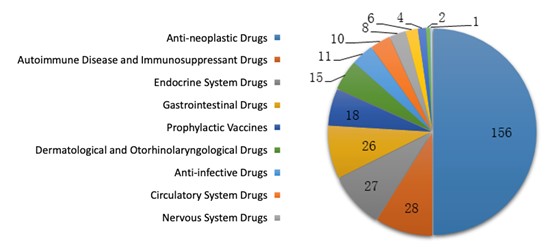
For biological INDs, anti-neoplastic treatment was a hot topic in drug development and covered 50% of the approvals. Meanwhile, half of the biological NDA approvals were for anti-neoplastic and endocrine treatment.
The diagrams below (Fig. 11 and 12) show the number of approvals for each indication of biological IND and NDA registrations respectively in 2019.
Accestra Consulting Provides China Regulatory Affairs Outsourcing for:
- Drugs/Pharmaceutical,
- Food and Feed products for China NMPA (Formerly CFDA),
- Regulatory approval including product registration for China Investigational New Drug (IND),
- China New Drug Application (NDA),
- China Drug Master File (DMF) for APIs,
- Excipient and Packaging Materials,
- ANDA, eCTD submission and others.
For queries, please contact us at: info@accestra.com
[wpuf-registration]








