China Drug Master File Overview
The scope of China Drug Master File (DMF) filing covers:
- Active Pharmaceutical Ingredients (APIs),
- Pharmaceutical excipients and packaging materials.
The registration approval by China Food and Drug Administration (CFDA) was replaced with a new DMF filing system.
It requires overseas manufacturers to appoint an authorized agent in China for product registration with National Medical Products Administration (NMPA formerly CFDA).
China DMF Filing Process
- To register for DMF filing you need to submit an application dossier to China’s Center for Drug Evaluation (CDE).
- They will review your application for documentation completeness and you will get an inactive filing number within 5-10 working days.
- The dossier will then be reviewed together with the dossier of the related finished dosage form (Drug-related Associated Review) to get the activated filing number.

Does China DMF comply with US FDA or EU EMA Requirements?
China DMF filing is more similar to the US FDA although it does reference some similarities with the EMA regulations.
Unlike the EMA regulations, the China NMPA and US FDA regulations don’t have open or closed/restricted parts.
Like US FDA, China NMPA adopts a negative approval system neither approving nor disapproving a DMF filing.
Although DMF filing guidelines of China are very aligned to that of developed countries since China NMPA joined the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) in 2017, some requirements need to be localized under the general regulatory framework for drugs.
China DMF Summary
China Product registration of APIs, pharmaceutical excipients, and packaging materials have reformed over the years.
After adopting the new DMF filing system, the license of marketing authorization has changed from import drug license to (active) filing number.
The regulatory requirements for application dossiers and workflow of review and approval has been revised.
This benefits overseas manufacturers by minimizing their budget and time cost for product registration.
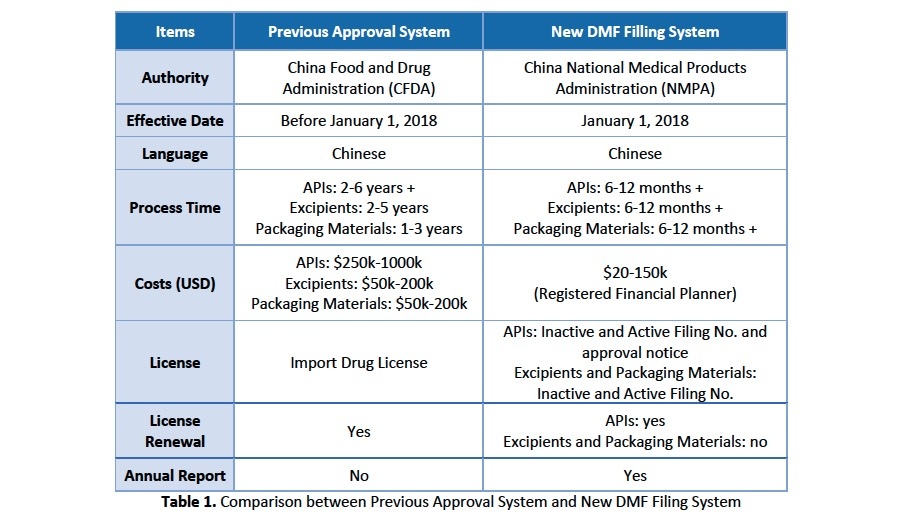
View the table below for a more detailed comparison between the previous approval system and the new DMF filing system (Table 1).
DMF Policy Update (August 2019)
On July 16, 2019, China NMPA published the new DMF requirements (Announcement No. 56 of NMPA, 2019), which was a major update to DMF filing for APIs, pharmaceutical excipients & packaging materials.
Key takeaways of the new requirements are below:
- Risk-based evaluation for excipients and packaging materials.
- Annual report being submitted every first quarter of the year.
- List of excipients no longer required.
- Previously approved APIs in China are exempted from drug-related associated review.
- Dossier requirements and workflow simplified.
- Find out more about the DMF policy update.
Is China DMF ECTD Ready?
In 2018, CDE established an e-platform for submitting DMF filing for:
- APIs,
- Pharmaceutical excipients,
- Packaging materials.
The platform was recently updated and is now more friendly to use.
But, becoming a member of the ICH meant China’s technical requirements for pharmaceutical products needed to conform with international guidelines.
As a result, NMPA has released new regulations for CTD/eCTD submission in the past two years.
From February 1, 2018, application dossiers for clinical trial approval, chemical drug and therapeutic biological product registration need to be submitted in CTD format.
In 2019, specifications, validation criteria, and submission requirements were released by NMPA for eCTD submission.
References:
Announcement No. 146, 2017 (published by the former China Food and Drug Administration (CFDA))
Announcement No. 56, 2019 (issued by China National Medical Products Administration (NMPA))








