In 2019, the Center for Drug Evaluation (CDE) of China reviewed and approved a total of 8730 drug registration applications.
As a follow-up on part 2 of Drug Evaluation Report 2019, part 3 further gives an overview of drug approvals for all application types (incl. IND registrations), drug priority review & accelerated approvals, and drug registration supporting activities, such as NMPA on-site inspection/audit, CDE meetings, and technical issue Q&As.
1. Drug Approvals
1.1 Administrative Approval
In 2019, CDE administratively approved a total of 5983 registration applications for chemical drugs, traditional Chinese medicines and biological products (excl. registrations for provincial review), among which technical review was required for 4075 registration applications and the others (supplementary applications and approvals for temporarily imported drugs) could be approved directly.
1.1.1 Technical Review & Administrative Approval
Among 4075 registration applications reviewed and approved by CDE, the number of approvals for each application type is listed in Table 1 below.
| APPLICATION TYPE | NUMBER OF APPROVALS |
|---|---|
| IND (incl. Bridging Studies) | 1124 |
| Consistency Evaluation | 345 |
| Supplementary Application | 2127 |
| Re-registrations of Imported Drugs | 471 |
| Review of re0application | 8 |
| TOTAL NUMBER |
Table 1. Number of Administrative Approvals for each application type in 2019.
According to the regulatory requirements for IND registration, IND approvals were issued by default within 60 days upon positive results of technical review by CDE. Including the approvals issued during the technical review of ANDAs for supplementary clinical trials, CDE issued a total of 1178 notifications of clinical trials in 2019. They were 1066 clinical trial (IND) approvals and 112 suspension notifications of clinical trials.
1.1.2 Direct Administrative Approval (No Technical Review)
Among the approved registration applications, 1908 applications were approved directly by NMPA or CDE without being technically reviewed, including 1491 supplementary applications and 417 approvals for temporarily imported drugs.
The average timeline of administrative approval was 9.9 workdays, whereas the stipulated timeline was 20 workdays of each approval.
1905 Applications were approved within the stipulated timeline, which gave 99.8% of on-time completion of the year.
1.2 Priority Review & Accelerated Approval
1.2.1 Priority Review
In order to reduce the backlog of registration applications and promote drug innovations, China Center for Drug Evaluation (CDE) accepted 253 applications for priority review of drug registrations in 2019, representing a decline of 19.2% from the last year.
The table below (Table 2) shows the number of applications accepted for priority review under each registration classification/application type from 2016 to 2019.
The most applications accepted for priority review in 2019 were registrations for new drugs with substantial clinical benefit (34%) followed by parallel registration applications (28.1%).
Comparing to the previous year, the number of registration applications for new drugs with substantial clinical benefit increased from 23% to 34%, whereas the number of re-applications for generics after consistency evaluation decreased from 16.6% to 7.9%.
As indicated by the data, the number of generic registrations has declined continuously so that more and more registrations of innovative new drugs with substantial clinical benefit or urgent medical needs could be reviewed with priority.
1.2.2 Drug Accelerated Approval
There were 143 registration applications (82 drug categories) approved for priority review and issued with marketing authorizations with acceleration, such as chemical drug Class 1 Innovative New Drug, i.v. Remimazolam Tosylate and Sodium Oligomannate Capsules. The table below (Table 3) shows the number of drug categories of accelerated approvals from 2016 to 2019.
The drug category of a chemical drug is referred to as the active pharmaceutical ingredient for the making of that drug, whereas the drug category of a biological product or traditional Chinese medicine is referred to as the international nonproprietary name (INN) of that drug.
| REGISTRATION APPLICATION | 2016 | 2017 | 2018 | 2019 | ||||
|---|---|---|---|---|---|---|---|---|
| Number of applications | Ratio | Number of applications | Ratio | Number of applications | Ratio | Number of applications | Ratio | |
| New Drug with Substantial Clinical Benefit | 85 | 44% | 106 | 46.10% | 72 | 23% | 86 | 34% |
| Parallel Application | 19 | 9.80% | 36 | 16% | 86 | 27.50% | 71 | 28.10% |
| Orphan Designation for Rare Disease | 8 | 4.10% | 11 | 5% | 28 | 8.90% | 28 | 11.10% |
| Pediatric Drug | 17 | 9% | 30 | 13% | 35 | 11.20% | 24 | 9.50% |
| Re-Application after Generic Consistency Evaluation | – | – | 10 | 4% | 52 | 16.60% | 20 | 7.90% |
| Major National R&D Project Drug | – | – | – | – | 15 | 4.80% | 19 | 7.50% |
| Generic of Coming-off Patent Drug | 16 | 8% | 18 | 8% | 25 | 8% | 4 | 1.60% |
| Drug with Urgent Medical Need or of Market Shortage | 5 | 3% | 12 | 5% | – | – | 1 | 0.40% |
| First Generic Drug Approval | 43 | 22% | 7 | 3% | – | – | – | – |
| IN TOTAL | 193 | 100% | 230 | 100% | 313 | 100% | 253 | 100% |
Table 2. Number of Applications Accepted for Priority Review from 2016 to 2019.
2. Communication and Meeting with CDE
2.1 Overview
In order to guide applicants through drug registration and further improve the support for drug innovations, CDE increased the number of communication channels and improved communication effectiveness in 2019.
These communication channels include meetings, Q&As for general technical issues, consultations by telephone, E-mails and on-site communication.
In the new model of multi-channel communication, 2633 applications were received for CDE meetings with an increase of 32.8%, among which 1871 applications were approved with an increase of 41.1%.
On the internet platform of CDE, 16572 questions were received for general technical issues with an increase of 8.9%.
The figures below (Fig. 1 and 2) show the number of applications respectively for CDE meetings and general technical issue Q&As from 2016 to 2019.
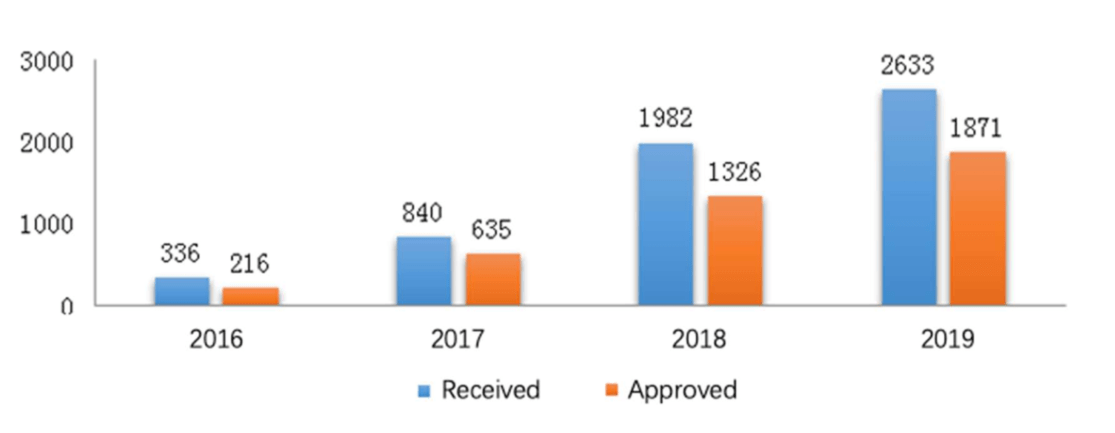
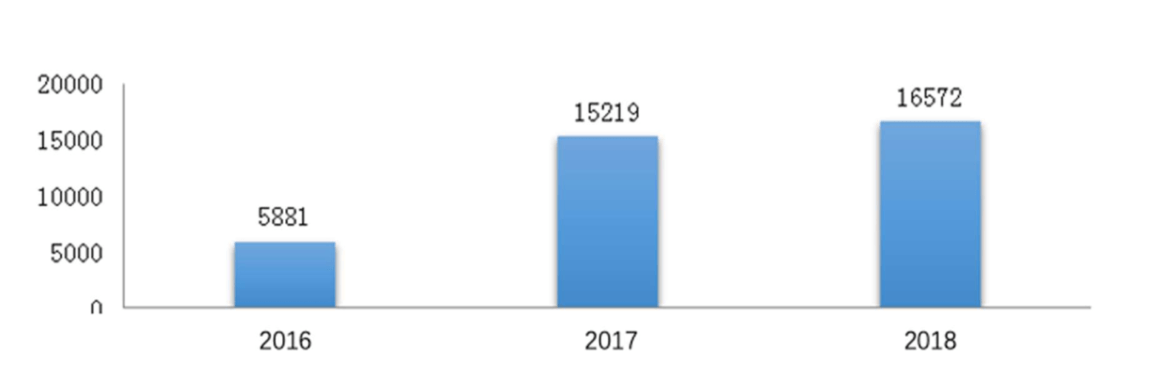
2.2 CDE Meetings
Among 1871 approved applications for CDE meetings, Class II meetings shared 71.8% of total approvals. The most requested Class II meeting was pre-IND meeting with a ratio of 34.9% of all approved meetings. An overview of all approved CDE meetings is given in the table below (Table 4).
| CLASSIFICATION OF CDE MEETINGS | NUMBER OF APPLICATIONS | NUMBER OF APPROVALS | RATIO OF APPROVAL | |
|---|---|---|---|---|
| CLASS 1 | 77 | 49 | 63.60% | |
| CLASS 2 | Pre-IND Meeting | 1027 | 653 | 63.60% |
| IND Meeting | 205 | 159 | 77.60% | |
| End of Phase 1 Trial Meeting | 206 | 152 | 73.80% | |
| End of Phase 2 Trial Meeting | 182 | 146 | 80.20% | |
| Pre-NDA Meeting | 248 | 193 | 77.80% | |
| NDA Meeting | 44 | 30 | 68.20% | |
| Generic Consistency Evaluation Meeting | 6 | 3 | 50% | |
| Complex Generic Consistency Evaluation Meeting | 13 | 8 | 61.50% | |
| Class 3 | 625 | 478 | 76.50% | |
| IN TOTAL | 2633 | 1871 | 71.10% |
Table 4. Overview of CDE Meeting Applications approved in 2019.
When holding meetings, CDE arranged either face-to-face (F2F) meetings or e-conferences through video or phone call. In 2019, 421 meetings were held with an increase of 30.7%. The table below (Table 5) shows the number of CDE meetings being held under each classification from 2018 to 2019.
| CLASSIFICATION OF CDE MEETINGS | 2018 | 2019 | |||
|---|---|---|---|---|---|
| NUMBER OF HELD MEETINGS | RATIO | NUMBER OF HELD MEETINGS | RATIO | ||
| CLASS 1 | 20 | 4.80% | |||
| CLASS 2 | Pre-IND Meeting | 102 | 37.30% | 134 | 31.80% |
| IND Meeting | 31 | 9.60% | 33 | 7.80% | |
| End of Phase 1 Trial Meeting | 37 | 11.50% | 33 | 7.80% | |
| End of Phase 2 Trial Meeting | 47 | 14.60% | 42 | 10% | |
| Pre-NDA Meeting | 87 | 27% | 71 | 16.90% | |
| NDA Meeting | 6 | 1.40% | |||
| Generic Consistency Evaluation Meeting | 1 | 0.20% | |||
| Complex Generic Consistency Evaluation Meeting | 2 | 0.50% | |||
| Class 3 | 79 | 118.80% | |||
| IN TOTAL | 322 | 100% | 421 | 100% |
Table 5. Number of CDE Meetings being held from 2018 to 2019.
2.3 General Technical Issue Q&As
Among 16572 questions received for general technical issues on the platform of CDE, most questions were for:
- Active Pharmaceutical Ingredients (APIs),
- Excipients and packaging materials (4152 questions),
- Followed by application acceptance of drug registration (1846 questions).
Chemical drugs remained the most concerning products for drug registration (9743 questions) with a majority of questions of generic consistency evaluation (1386 questions) and application acceptance (1174 questions).
For an overview of general technical issue Q&As in 2019, please find more details in the table below (Table 6).
| CLASSIFICATION OF Q&As | API, Excipient, Packaging | Chemical Drug | Traditional Chinese Medicine & Natural Medicine | Biological Product | Others | Total Numbers |
|---|---|---|---|---|---|---|
| API, Excipient, Packaging | 2873 | 1155 | 22 | 81 | 21 | 4152 |
| Applicaiton Acceptance | 134 | 1174 | 77 | 327 | 134 | 1846 |
| Technical Review – CMC | 27 | 1176 | 39 | 485 | 12 | 1739 |
| Generic Consistency Evaluation | 16 | 1386 | – | 5 | 33 | 1440 |
| Technical Review – Clinical Trials | – | 854 | 67 | 304 | 38 | 1263 |
| Technical Review – Regulatory Compliance | 30 | 814 | 78 | 196 | 61 | 1179 |
| Guidelines | 37 | 397 | 55 | 120 | 61 | 670 |
| Material Supplement | 81 | 482 | 29 | 69 | 9 | 670 |
| Internet Platform of CDE | 134 | 133 | 10 | 17 | 68 | 362 |
| Technical Review – Pharmacology, Toxicology | 169 | 17 | 112 | 3 | 301 | |
| Technical Review – Statistics/Clinical Pharmacology | – | 86 | 2 | 15 | 6 | 109 |
| Others | 143 | 1917 | 119 | 396 | 266 | 2841 |
| In Total | 3475 | 9743 | 515 | 2127 | 712 | 16572 |
Table 6. Number of Q&A’s for General Technical Issues in 2019.
3. NMPA On-site Inspection/Audit
In order to comprehensively review drug registration applications, the National Medical Products Administration (NMPA) of China conducted on-site inspections/audits not only for general issues but also specific problems which were reported to NMPA (“For-cause” Inspection).
The table below (Table 7) shows the number of tasks and reports achieved respectively for general and “for-cause” inspections/audits in 2019.
| GENERAL INSPECTION | ||||
|---|---|---|---|---|
| CLASSIFICATION | CMC | Clinical Trials | Pharmacology & Toxicology | Total Number |
| Number of tasks | 782 | 446 | 2 | 1230 |
| Number of reports | 689 | 551 | 2 | 1242 |
| “FOR-CAUSE” INSPECTION | ||||
| Number of tasks | 12 | 12 | ||
| Number of reports | 8 | 8 |
Table 7. Overview of on-site inspection & audit in 2019.
Accestra Consulting Provides China Regulatory Affairs Outsourcing for:
- Drugs/Pharmaceutical,
- Food and Feed products for China NMPA (Formerly CFDA),
- Regulatory approval including product registration for China Investigational New Drug (IND),
- China New Drug Application (NDA),
- China Drug Master File (DMF) for APIs,
- Excipient and Packaging Materials,
- ANDA,
- eCTD submission and more.
For queries, please contact us: info@accestra.com
[wpuf-registration]