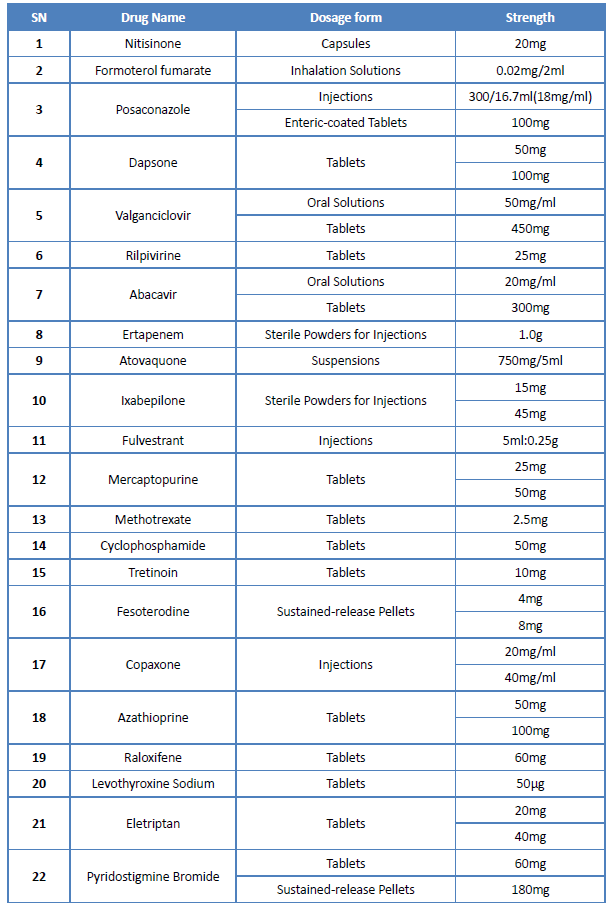
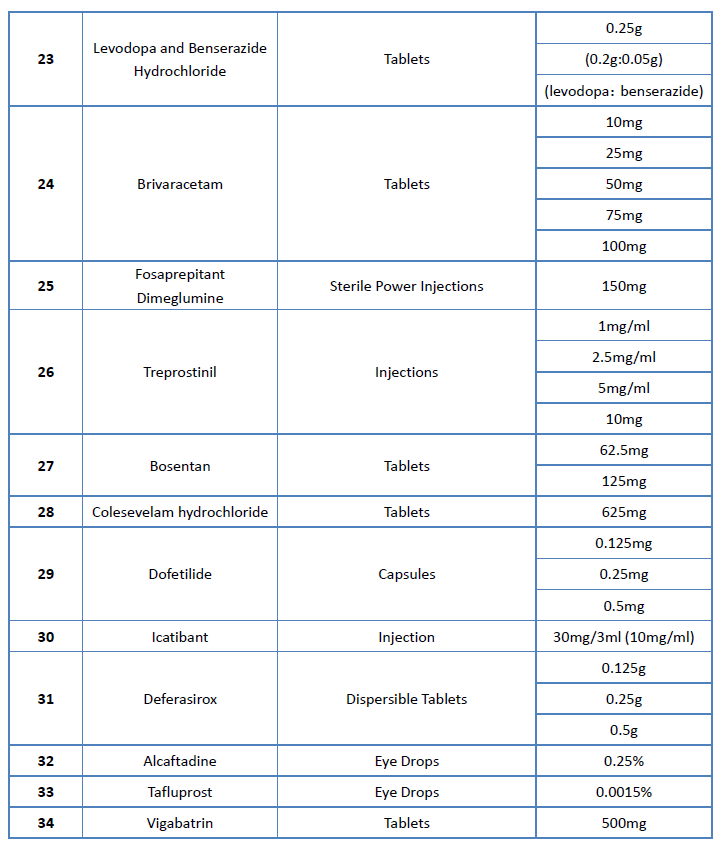
On June 20th, China National Health Commission (NHC) officially announced the draft list of 1st batch of encouraged generic drugs (NHC is a ministry-level institute of the State Council, in which the Department of Drug Policy and Essential Medicine takes the responsibility of improving the national essential medicine system and establishing national drug policies and Essential Drug Catalogue). There are a total of 34 drugs in this drafted list, including drugs came off patent (or are about to expire) in China but no MA application is submitted, and drugs in short supply in clinical.
The release of this list is considered as the follow-up of two previous important documents on generic drugs supply issued by China authority in 2018, one of which is the “Opinions on Reforming and Improving the Supply and Protection Policy of Generic Drugs” by the General Office of the State Council, and the other is the “Notice of Printing and Distributing the Work Plan for Accelerating the Implementation of Generic Drugs Supply Guarantee Policy” issued by NHC and other 12 ministries. In these two documents, it is proposed that China will regularly publish lists of encouraged generic drugs: before the end of June 2019, the first list will be released; from year 2020 onwards, such list will be released at the end of each year. And according to the “Notice”, for drugs in these encouraged lists, the priority review and approval pathway shall be applicable in the process of MA application to China NMPA.
So far it’s the 1st batch of list issued by China NHC, and the publicity time is 5 working days. However, the corresponding favorable policy details for the listed drugs have not been announced yet.