The beginning of June has seen the release of 2021 Drug Review Annual Report by China’s Center for Drug Evaluation (CDE) of National Medical Products Administration (NMPA).
In 2021, the drug review work has achieved new records by accomplishing 47 innovative new drug approvals.
In addition, the completion rate of on-time review & approval of registration applications has increased to 98.93%, with historical breakthroughs in multiple categories of registration applications.

compiled key highlights below:
-
A Growing Number of Accepted Registration Applications
- In 2021, CDE has accepted 11,658 registration applications, a year-on-year growth of 13.79%. The number of registration applications requiring technical review was 9235, an increase of 29.11% year on year, and 2423 registration applications were subject to administrative approval directly without technical review.
- The number of registration applications for Active Pharmaceutical Ingredients (APIs) reached 1313, up by 2.98% from last year.
- Category Application Breakdown
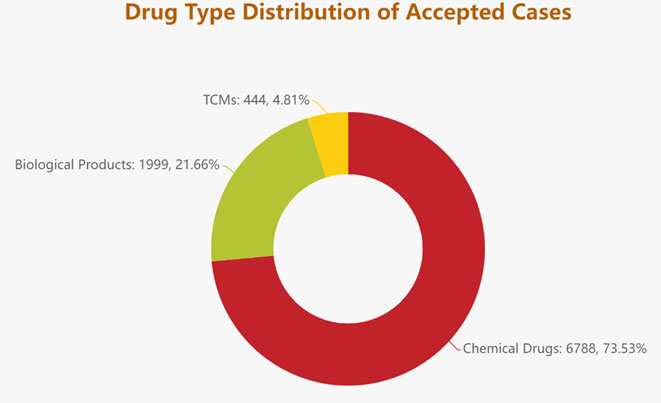
- Amongst the registration applications that required technical review, a breakdown from drug types is as follows:
- To see from categories of registration applications, CDE accepted 2412 applications for Clinical Trial for New Drug (hereinafter referred to as IND), spiking 55.81% of last year. The number of applications for marketing authorization of New Drugs (hereinafter referred to as NDA) reached 389, growing 20.43% year on year.
-
A Surge in Completed Review & Approval
- In 2021, CDE has completed the review & approval of 12,083 registration applications, rising by 19.55% year on year.
- CDE closed 9679 registration applications requiring technical review, a rise of 35.66% of last year—including 2632 registration applications with technical review, 7039 for review & approval, and 8 for drug-device combination. 2404 registration applications were directly approved.
- By the end of 2021, 5652 cases were still under review & approval, with 1353 cases awaiting supplementary materials from applicants.
- For APIs, CDE has completed review & approval of 494 cases, holding 1302 registration applications under review or approval and 582 applications for supplementary materials by the end of 2021.
- Category Application Breakdown
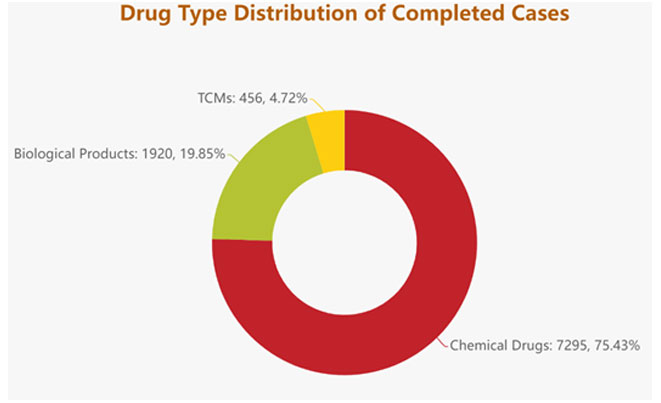
- In terms of drug types, amongst the completed registration applications that required technical review, TCM, Chemical Drugs, and Biological Products have all remarkably increased, with 22.25%, 34.22% and 45.12% respectively. The detailed category breakdown is as follows:
For more detailed information about China’s CDE drug approval or any questions, please contact us at info@accestra.com .