On January 7th, 2020, NMPA promulgated “Guiding Principles of Real World Evidence supporting Drug Development and Review (Trial)”. It introduces the background and purpose of Real-World Research/Study (RWR/RWS), relevant definitions, the role of Real-World Evidence (RWE) in drug regulatory decision, the basic design of RWS, the evaluation of RWE and communication with review agencies. This guiding principle defines the status and role of RWS and is of great significance to the development of China’s RWS.
1.Relevant definitions of RWS
RWS refers to the pre-set clinical problems, which is the study process of collecting and studying the data (Real-World Data, RWD) related to research subject’s health or the summarized data derived from these data in the real-world environment, and obtaining the status of drug application and the clinical evidence (RWE) of potential benefits-risks through analysis work (as the figure below shown).
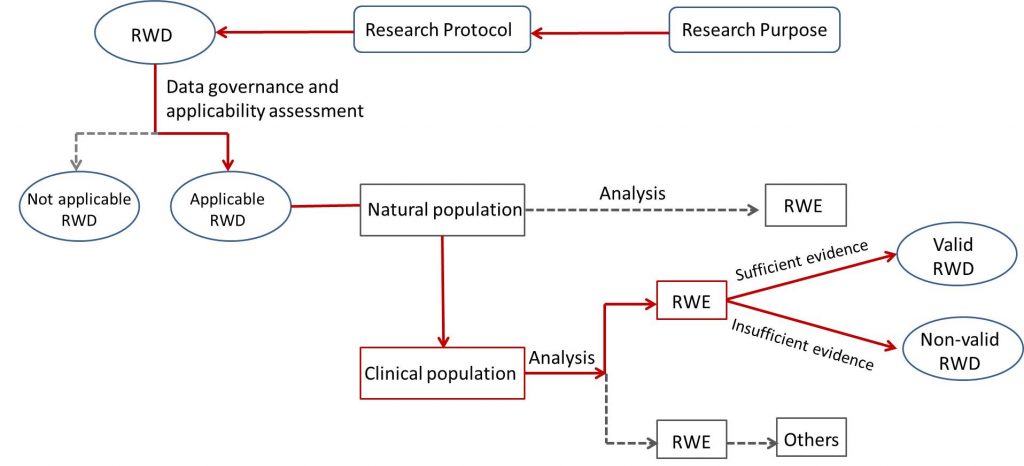
RWD refers to a variety of data collected on a daily basis related to patients’ health conditions and/or diagnosis, treat and healthcare. Not all the RWD could be RWE after analysis, only the RWD meet applicability may produce RWE.
RWE refers to the clinical evidence on the status of drug application and potential benefits-risks obtained through the appropriate and sufficient analysis of the applicable RWD, including the evidence obtained through intervention studies, such as Retrospective Observational Study or Prospective Observational Study or Pragmatic Clinical Trial (PCT) etc.
2. RWE supports drug regulatory decisions
RWE is applied to support drug regulatory decisions, it covers many steps of drug research including pre-market clinical development and post-market re-evaluation.
(1) Provide evidence of efficacy and safety for new drug registration and marketing
According to the characteristics of different diseases, access to therapeutic methods, target population, therapeutic effect and other factors related to clinical research, the efficacy and safety information of drugs can be obtained through RWS, which provides supportive evidence for new drug registration and marketing.
The common real-world studies provide the evidence of efficacy and safety for new drug registration and marketing are: the randomized controlled trials (RCT) with outcome or safety data obtained from RWD, including PCT design, etc; and the single-arm trials based on the RWE as external control, for some rare diseases and serious diseases threaten life lack of effective therapeutic treatments.
(2) Provide evidence for changes of the introduction of marketed drugs
For marketed drugs, new indications generally require RCT support. However, when the RCT is not feasible or the research design is not optimal, it may be more feasible and reasonable to use the RWE generated by PCT of observational study to support the new indications.
In the area of pediatric drugs, using RWE to support the expansion of indication population may be also one of the applicable situations for drug regulatory decision.
In general, RWE supports the changes of instructions of marketed drugs mainly include:
- Add or modify indications;
- Change dosage & administration regimen or routes of administration;
- Add new applicable population;
- Add the results of comparative effectiveness research;
- Add information on safety;
- Other modifications of instructions.
(3) Provide evidence for the requests and re-evaluation of marketed drugs
The drugs approved based on RCT evidence, usually have deficiencies like the limit of safety information, the uncertainty of extrapolation of efficacy conclusions, administration regimen may not be optimal and lack of economic benefits due to limited cases, short research time, strict condition of subject enrollment and intervention standardization. It is necessary to use RWD to conduct a more comprehensive evaluation on effectiveness, safety, use and economic benefits of drugs in real medical practice, and make decisions adjustment according to RWE continuously.
(4) RWE’s other applications used for regulatory decision
- Guide clinical study design
- Locate target population precisely
3. Evaluation of RWE
Evaluation of RWE shall comply with two main principles:
Whether RWE can support the clinical problems that need to be answered or not.
Whether the existing RWD can be scientifically designed, rigorously organized and implemented, and reasonably analyzed to obtain the required RWE.
(1) RWE and their supporting clinical problems
Before determining to use any evidence including RWE, the clinical problems need to be answered shall be defined first. For example, safety considerations for the combination of a marketed drug with other drugs; new indication researches for approved products; and the establishment of robust and reliable history or external controls for single-arm clinical trials of a rare disease, etc.
Secondly, it is necessary to consider whether the use of RWE can answer the facing clinical questions. Four aspects shall be evaluated based on scientific validity (for example, scientific interpretability, reasonability of hypothesis and type I error control, etc.), regulatory requirements (whether they conflict with other regulatory requirements, and whether there’s any regulatory requirement in the areas of special disease, etc.), ethical issues (whether ethical issues will be caused without using RWE) and operability (for example, whether there are independent statisticians and ensure that the statisticians are blind to outcome variables to avoid possible bias when matching; whether there’s any other operational challenge, etc.).
The comprehensive consideration of the above issues is an important criterion to measure the application of RWE.
(2) How to get to RWE from RWD
Basically it’s necessary to consider: ① The research environment and data collection are close to the real-world, such as more representative target population, the diversification of intervention accord with clinical practice, and natural choices of intervention, etc. ② Suitable control. ③ More comprehensive evaluation of results. ④ Effective bias control, such as the use of randomization, the unification of measurement and evaluation method, etc. ⑤ Appropriate statistical analysis, such as correct use of casual inference methods, reasonable processing of missing data and sufficient sensitivity analysis, etc. ⑥ The transparency and reproducibility of evidence. ⑦ Reasonable explanation of results. ⑧ All the parties concerned reach a consensus.
It’s important to note that all research designs, hypothesis and specific definitions related to the generation of RWE shall be stated clearly in the research protocol beforehand. The afterwards supplementary data reference, definitions, analyses and explanations normally cannot be used for regulatory decisions.
4. Communicate with review agencies
It’s necessary to communicate adequately with drug review department when using RWE for the purpose of drug registration, to ensure the two parties has reach a consensus on using RWE and conducting RWS.
When the applicant plans to use RWE to support drug registration matters, it shall apply for communication actively in accordance with the communication channels of drug review department before implementing researches. And perform written or conference communication and discussion from the feasibility of using RWE, research design, data collection and analytical methods.
After completing RWS, the applicant also shall apply for communicating with review department before planning to submit application materials, to communicate and confirm the contents of the research implementation status, research result and conclusion, the requirements of application materials.
This article is translated from the published post of NMPA.








