1. History of special review and approval
a. Initial definition: In the revised “Drug Registration Administration Law” of 2005, added special approval of “drugs necessary for emergencies”. This revised version was implemented on May 11th, 2005.
b. Issuance of review and approval procedure: “Drug special review and approval procedure of State Food and Drug Administration” was approved and promulgated by the council of SFDA (former NMPA) on November 18th 2005, and implemented since November 18th 2005.
This is the order No.21 of SFDA, which stipulates detailed procedures as follows:
- After drug special approval procedure is activated, SFDA shall be uniformly responsible for the acceptance of the registration applications of drugs needed for prevention and treatment of public health emergencies.
- SFDA will only comment on the scientific nature and feasibility of project of drug application, and respond within 24 hours.
- Special expert group shall proceed evaluation and review on the registration applications of drugs needed for prevention and treatment of public health emergencies, and decide whether to accept or not within 24 hours.
- SFDA shall organize to technical review within 24 hours, notify the provincial agency of the applicant to conduct on-site inspections and proceed sampling and test of samples.
c. The 2019 new reversion of “Drug Administration Law” has related provisions in Article 26 and Article 96:
Article 26: For drugs that treat diseases threaten life severely, have no effective treatment methods and urgently needed by public health, can be approved with conditions and state relevant matters in drug registration certificates if data of drug clinical trials already show efficacy and also predict its clinical value.
Article 96: The state encourage development and manufacture of drugs which are in short supply. These drugs in short supply which is clinical urgently needed and new drugs that can prevent and treat major infectious diseases and rare diseases shall be given priority review and approval.
d. In the revised draft (draft for comments) of “Drug Registration Administration Law” of 2019, a chapter entitled “Accelerate Drug Approval Registration” was added, the details as follows:
- Article 1 (Special review and approval situation) In case of a threat to a sudden public health emergency occurs and after the occurrence, NMPA may decide in accordance with law and implement special review and approval for the drugs urgently needed by preventing and treating sudden public health emergency events.
- Article 2 (Special review and approval policy) For drug registration applications subjected to special review and approval, NMPA accelerate and synchronize the acceptance, review, inspection and test work according to the principles of unified command, early intervention, fast and high efficient, review and scientific approval. The situations, procedures, time limits and requirements etc shall be implemented based on the provisions of “Drug special review and approval procedure” (that is the previously mentioned order No. 21 of 2005).
- Article 3 (Requirements of special procedure) Drugs included in special review and approval procedure can be used within a certain period of time and ranges in accordance with the requirements of specific needs of disease prevention and control.
e. Specific cases of special review and approval:
- H1N1 vaccine: In 2009, it took only 5 days from the registration to approval of manufacture due to starting special review and approval procedure for the H1N1 vaccine of SINOVAC BIOTECH CO., LTD.
- IPV vaccine: In 2017, NMPA adopted special review and approval channel to review IPV vaccine, which led to it only took 35 days for BEIJING TIANTAN BIOLOGICAL PRODUCTS CO.,LTD from the acceptance of registration application of manufacture to issuing drug approval number and new drug certificate.
- Ebola virus vaccine: The recombinant Ebola virus vaccine jointly developed by Biological Engineering Research Institute of Academy of Military Medical Sciences and CanSino Biologics INC applied for manufacture in April 27th 2017, and was approved for marketing in October 19th 2017, which is also reviewed through special review and approval channel.
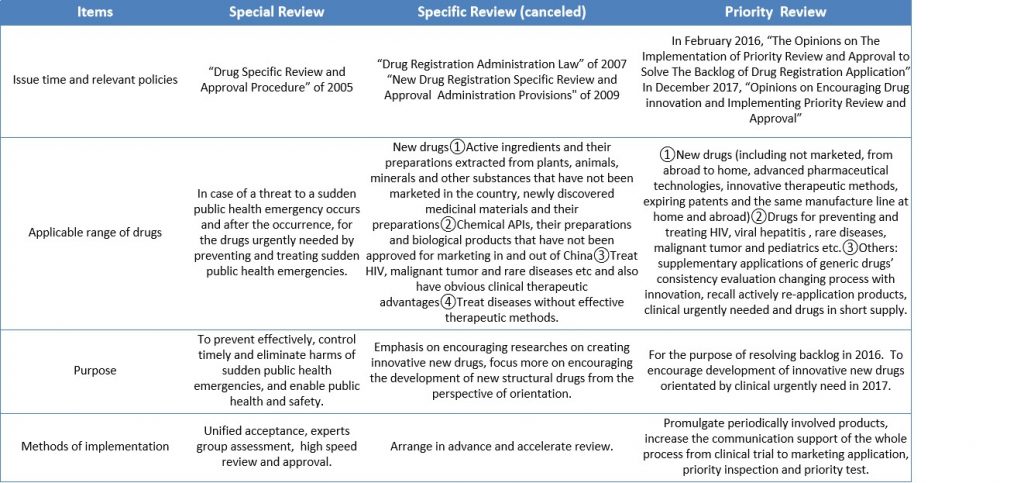
2. The differences between special review and approval and priority review and approval:
Table 1 Comparison of Special Review and Priority Review Mode
3. Special review and approval matters related to COVID-19
a. Free charge for drug registration through special review and approval procedure
The “Announcement on Exemption of Certain Administrative Fees and Government Funds During The Period of Preventing and Controlling Novel Coronavirus Infectious Pneumonia” issued by the Ministry of Finance on February 7th 2020, stipulated that products for emergency medical device review and approval procedure and 2019-nCoV related preventive and control products are exempt for medical device registration fee. And the drugs enter special review and approval procedure, for treating and preventing 2019-nCoV infectious pneumonia are exempt for registration fee.
b. Rapid detection reagents of NCP included in emergency review and approval procedure
On January 20th 2020, Center for Medical Device Evaluation of NMPA launched emergency review and approval work, and at the same time started to compile the points of product’s review and approval according to the requirement of “no reduction in standards, no decrease in procedures”. NMPA only took 11 days from deciding to start emergency review and approval procedure of medical devices to approving the first batch of 7 enterprises’ relevant products, of which the 4 products of former 4 enterprises getting approval only took 4 days.