In the year of 2019, CDE has accepted 6,106 application acceptance numbers of chemical drugs, 413 application acceptance numbers of traditional Chinese medicines and 1,078 application acceptance numbers of biological products (the data is calculated by acceptance number dimension, except for APIs, and the same below). The marketing application acceptance number of chemical drugs is 1,128, which is slightly more than that of 2018 and significantly higher than the number of 2017 (nearly third times). While the clinical application acceptance number is 829, which is obviously more than that of 2017 and 2018. Overall, the clinical and marketing application number are both more than that in 2017 and 2018.
In terms of approval, in 2019, the acceptance number has obtained review comment is 1,855. Among them, there are 834 approval clinical acceptance numbers, 427 approval manufacturing acceptance numbers and 99 imported acceptance numbers have been approved. Compared with the approved number in 2017 and 2018, the overall is in a fluctuation condition, which has a direct connection with the changes in registration approval policies and the increase of domestic review in recent years.
According to the statistics, it takes 427 days (50% shorter than 2018) for NDA and 1,205 days for ANDA (13% shorter than 2018). Among them, for imported new drug applications, the products has got priority review can be reviewed in 363 days, non-priority review products need 570 days to complete their reviews. However, it takes 422 days to complete the review of a domestic new drug has been given priority review.
1.Chemical drugs application statistic information in 2019
In 2019, 6,106 application acceptance numbers of chemical drugs involve 1,263 active ingredients and 1,470 enterprises. In the 6,106 numbers, the number of domestic chemical drugs is 3,256, accounting for 53%, and the imported chemical drugs application acceptance number is 2,850 which accounts for 47% (as the image below shown).
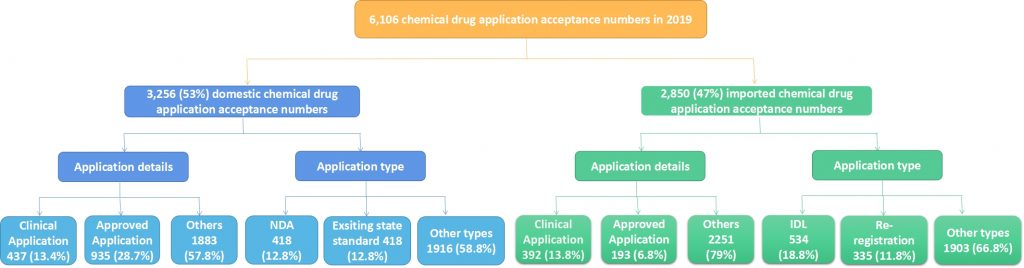
Note: Others in application content include: re-registration application, re-review, supplementary application and other applications. Other types include: supplementary application, re-review and other applications.
From the perspective of application type in recent 3 years, the NDA acceptance number has gradually increased and grown steadily, which has a close relation with China’s encouraging innovation policy. And the imported application acceptance number decreased slightly in 2018 has begun to increase substantially in 2019.
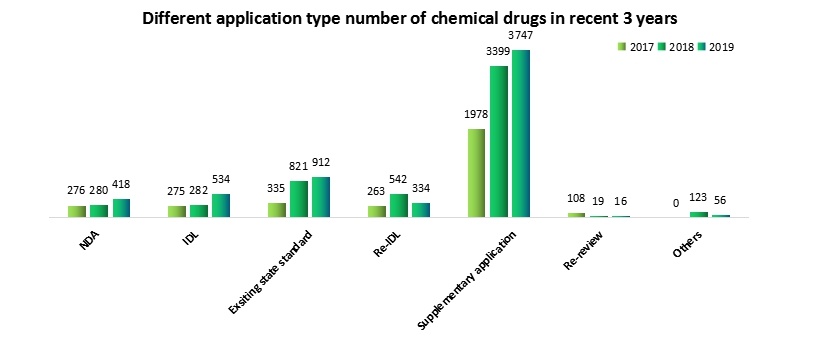
Note: Data based on Insight application progress database statistics to Dec. 31th 2019.
From the point of view of registration type, in 2019, there are 516 acceptance numbers of type 1 new drugs involving 221 products. In which, domestic type 1 NDA acceptance numbers are 332 involving 143 products, and 184 acceptance numbers of imported type 1 new drugs involving 78 products. For the improved type 2 new drugs, the number is 176 which involves 8 products. The number of type 3 and type 4 generic drugs are 221 (123 products) and 699 (201 products) respectively. And there are 271 acceptance numbers of type 5.1 and 129 acceptance numbers of type 5.2.
2. Chemical drugs approved statistic information in 2019
Among the 1,855 chemical drug acceptance numbers approved for CDE review and approval in 2019, domestic products acceptance numbers are 1,289, and import products acceptance numbers are 566. In terms of clinical approval, the number of clinical approvals started to increase in 2019 due to the negative comment clinical system, has reached a total of 827. In terms of marketing approval, 427 numbers are approved and 99 imported numbers are approved in 2019.
As of January 3 2020, the largest number approved of 2019 is supplementary application, followed by imported drug approval.

Note: The statistic time is Jan.3st 2020, statistics at different time points will cause differences in the number review comments. (This data is from Yaozhi)
This article is referenced and translated from Insight database and Yaozhi. We’ll get further detailed information at mid 2020 when CDE release official annual report of 2019.