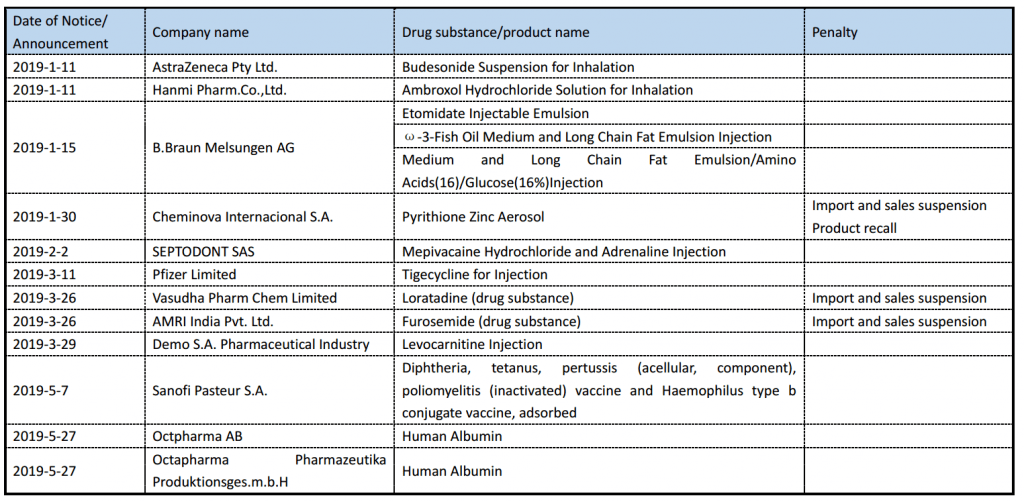
On December 26, 2018, China NMPA (National Medical Products Administration) released the Regulations for Drug and Device Overseas Inspection. Drugs and devices that have been approved or are applying for market in China are subject to this Regulation. The working procedure are summarized in the diagram below.
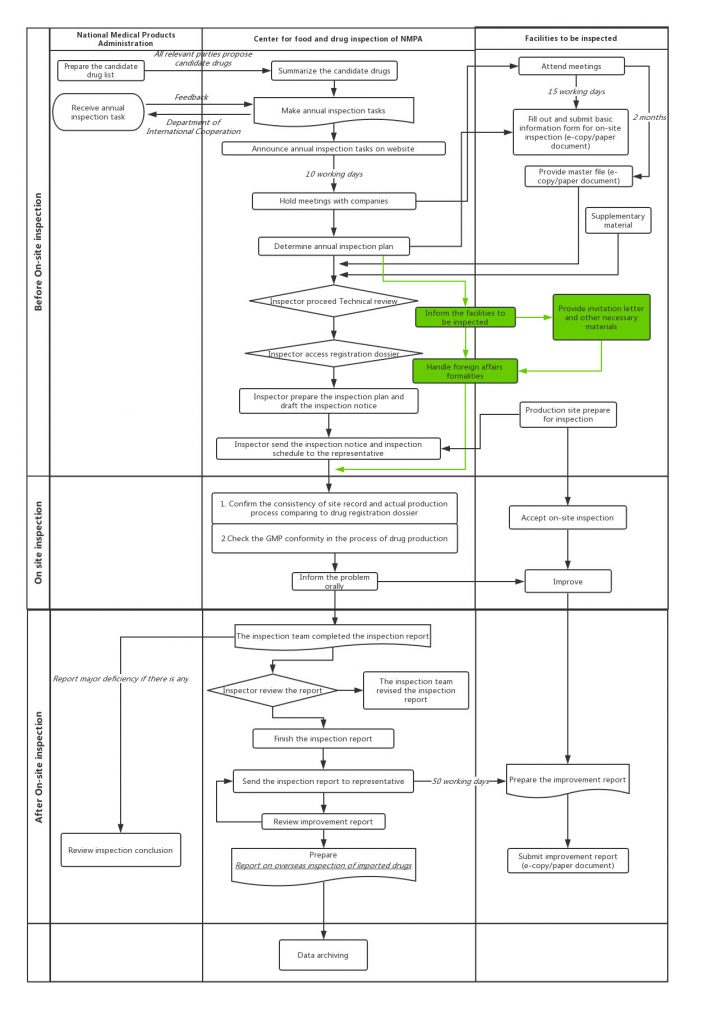
And in the first half of 2019, there are 12 drug inspection notices published on the website of Center for Food and Drug Inspection, NMPA, among which 3 companies are given the penalty of sales suspension in China. However, the imported drug manufacturers still experienced a much lower inspection frequency comparing to local companies. Although this new regulation can be considered as a signal that China authority wants to treat foreign companies and domestic companies equally, before that they still have the staff shortage problem to solve.