Since the Quality Consistency Evaluation (QCE) reform has been implemented nationwide in 2016, there are two questions all pharmaceutical companies in China facing: whether to initiate a QCE project? If yes, what kind of generic drug should be selected? And since the medical insurance policies for generics such as 4+7 (11 pilot Chinese provinces) hospital centralized procurement is getting much clearer recently, it seems the QCE is not only a race of company’s technical strength, financial muscle and project management competence, but most important the perception towards the entire Chinese pharmaceutical market.
Until the end of May 2019, the information published by CDE-NMPA shows that more than 1,100 QCE applications have been accepted after format review, involving over 340 pharmaceutical companies and more than 330 generic drugs. So which of them are the hottest ones?
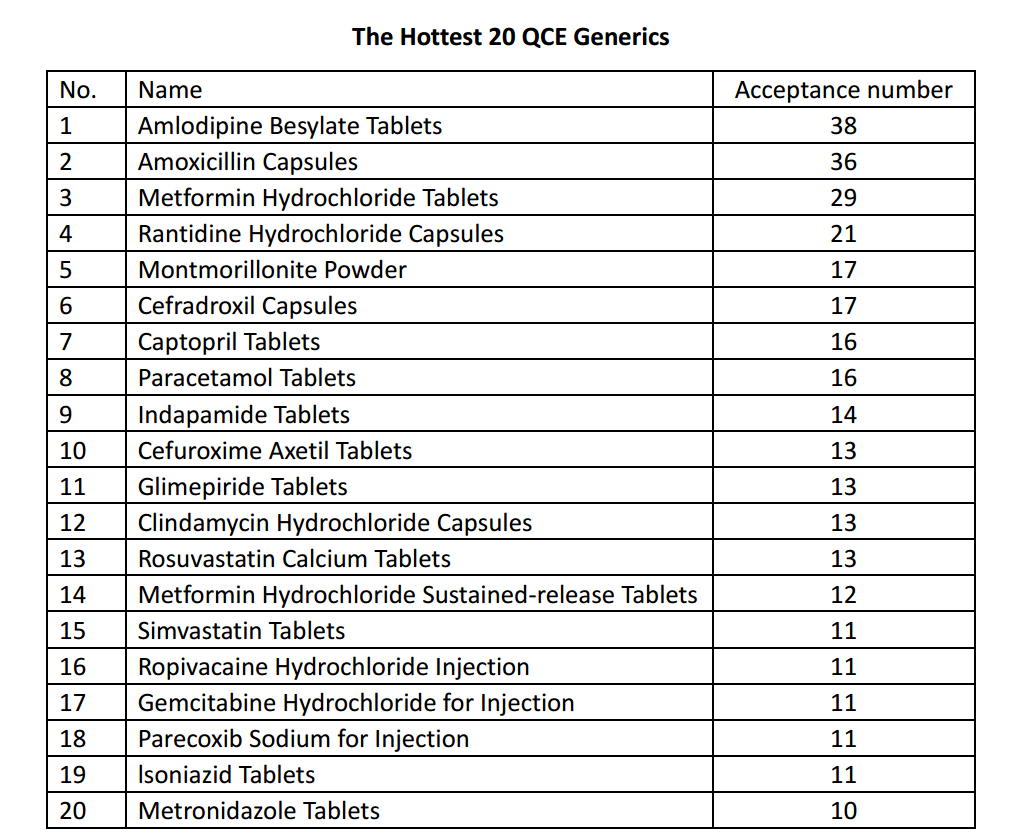
In addition, there are already more than 20 generic specifications which over 3 pharmaceutical company competitors have received regulatory approval of them under QCE system, such as Amlodipine Besylate Tablets, Tenofovir disoproxil fumarate tablets, Rosuvastatin Calcium Tablets and Metformin Hydrochloride Sustained-release Tablets, etc. However, according to the new centralized procurement policy (4+7) which has enforced in 11 pilot Chinese provinces, if there are more than 3 pharmaceutical companies providing the same drug approved under QCE system, the company whose drug has not complete the consistency evaluation will be no more selected as the supplier. Hence, for these hot generic drugs, the company without QCE approval has lost the chance to stand on the boxing ring, and for those companies who got their QCE tickets already, there is another round of price & marketing fight waiting for them.








