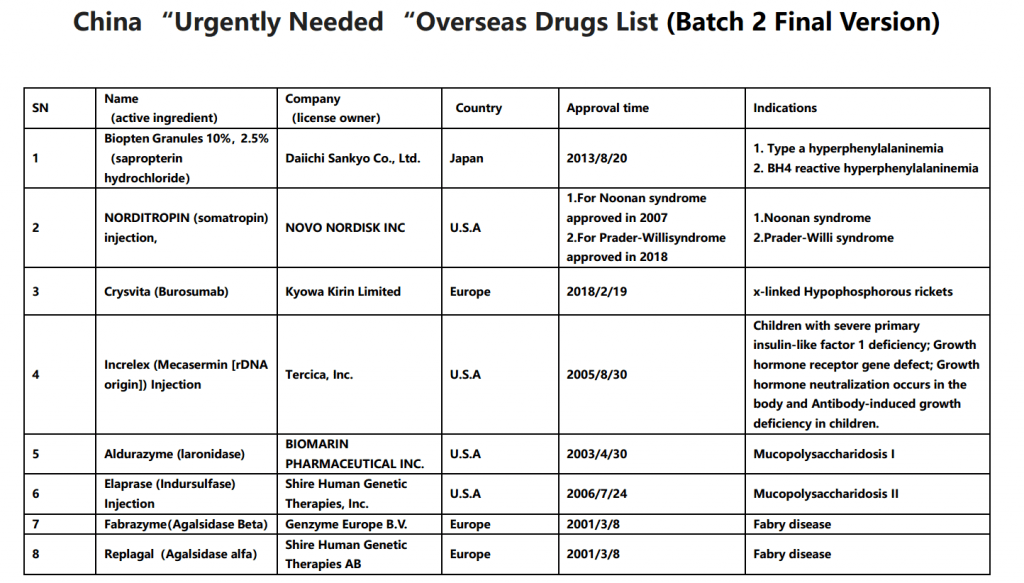
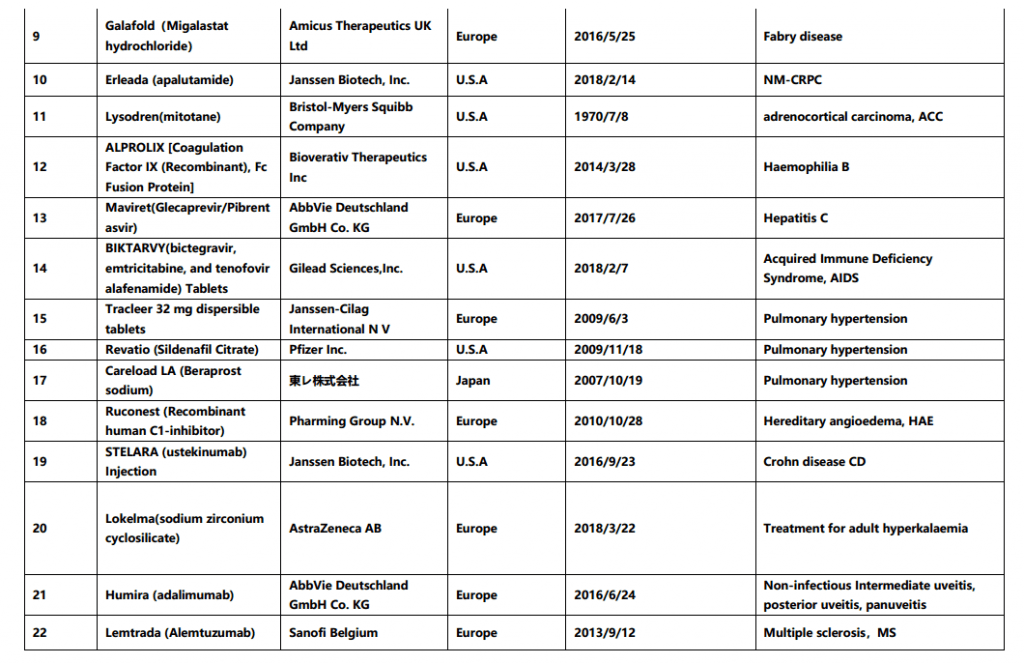
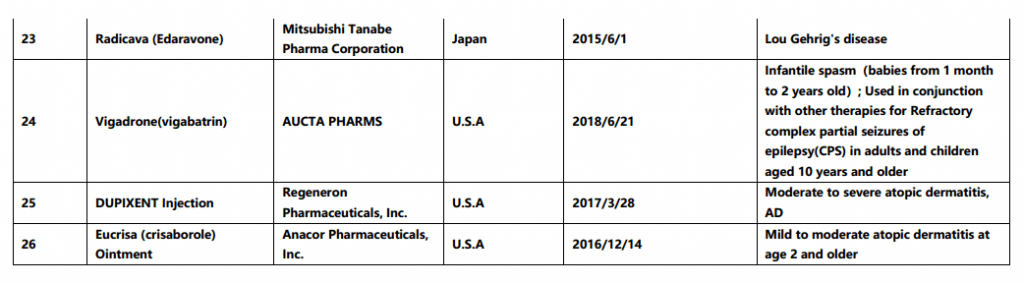
On May 29th, China Center for Drug Evaluation (CDE) released the second batch of overseas approved new drugs that are clinically urgently needed in China (final version), the list contains 26 drugs including Biopten Granules and more. Comparing with the draft list for public consultation given by CDE on March 28th 2019, below 4 drugs have been deleted in this final list:

As mentioned in our previous news given on 13th of April (details of previous news are accessible here), National medical products administration (NMPA) issued a notice (No. 79) on 31st of October 2018, which is “The review and approval process for urgent clinical needs foreign drug”, illustrating how the fast track procedures work for “urgently needed” drug, which can be divided into 3 conditions as follows:
- For drugs that the clinical trial application has not been applied yet, the applicant may apply for MA to CDE directly;
- For drugs that clinical trial application has previously submitted already but the technical review has not been completed, the applicant may submit a written application to the CDE to adjust the CTA to MA application, and supplement all the research data obtained abroad and relevant supporting materials to prove there is no racial differences.
- For the drugs that clinical trials are on-going, the applicant can apply to CDE for MA at the same time while continuing clinical trials. Upon completion of the clinical trial, the applicant should submit all research reports to the CDE in a supplementary application form
CDE will establish special technical review process for new drug in the “urgently needed” list: the technical review timeline can be shortened to 3 months for orphan drugs and 6 months for other new drugs, while the standard technical review time for new drug is normally 120-160 working days.