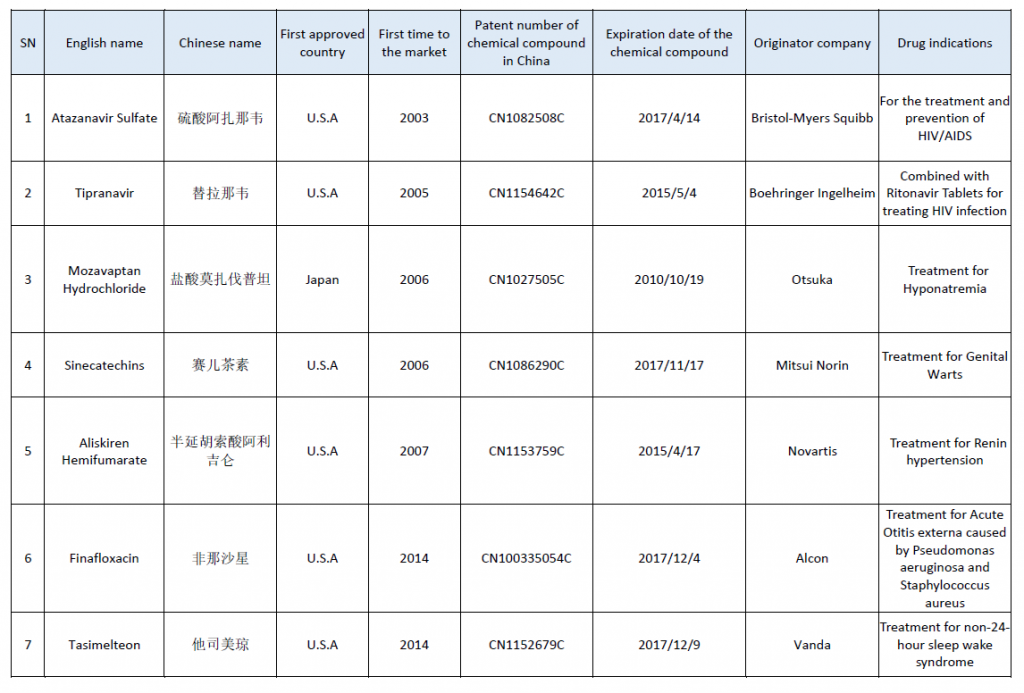
The second batch of off patent drugs published by China Center for Drug Evaluation (CDE) contains 7 abroad approved drugs, which are regarded as clinically valued and the patent of chemical compounds has been expired, invalid or terminated in China, however, there is still no generic drug application in China until October, 2018. The Anti-HIV drugs Atazanavir Sulfate with Chemical compound’s patent number CN1082508C is put on the top of the list, with the patent expiration date of 14th April, 2017. Although the number of AIDS patients in China is increasing, domestic pharmaceutical companies seem to have relatively little passion for the innovation and generic drug research of the therapeutic drugs, which is indeed worth attention.
Off-patent drugs published by China CDE (batch 2)