Part 3. Administrative Approval and Fast Track Review Pathways
Table of contents
|
In 2020 CDE approved:
This includes 59 drugs for the treatment of COVID-19.
As a follow-up on part 2 of the Drug Evaluation Report 2020, part 3 gives an overview of administrative drug approvals and fast track review pathways by CDE.
This includes specialized pathways, breakthrough therapy, conditional approval, and priority review.
|
1. Administrative Approval
CDE administratively approves a drug application regardless of whether it needs a technical review.
In 2020, there was a total of 8,646 approved applications, an increase of 44.51% year on year.
Most of these applications (5,647) were reviewed technically before approval, such as:
- Clinical Trial Applications (CTAs),
- New Drug Applications (NDAs),
- Abbreviated New Drug Applications (ANDAs),
- License renewal of imported drugs.
Other applications (2,972) were approved without technical reviews, such as temporary drug import applications.
Table 1 below gives an overview of applications administratively approved in 2020.
|
APPLICATION TYPE |
|
NUMBER OF APPROVALS |
|
|
|
|---|---|---|---|---|---|
|
|
CHEMICAL DRUGS |
BIOLOGICAL PRODUCTS |
TRADITIONAL CHINESE MEDICINE |
IN TOTAL |
|
| Approval after technical review | CTAs (incl. confirmatory clinical trials) | 1,085 | 564 | 37 | 1,686 |
| Consistency Evaluations | 623 | 0 | 0 | 623 | |
| Supplementary Applications | 1,955 | 615 | 290 | 2,860 | |
| License Renewal of Imported Drugs | 406 | 49 | 23 | 478 | |
| Re-Review | 17 | 3 | 7 | 27 | |
| Approval without technical review | Directly Approved Supplementary Applications | 2,048 | 348 | 141 | 2,537 |
| Temporary Import Application | 363 | 60 | 12 | 435 | |
| IN TOTAL | 6,497 | 1,639 | 510 | 8,646 |
Comparing to 2019, 39.24% more applications were approved after technical review and 55.77% more applications were approved directly (without technical review).
View the diagram below for a comparison between the last three years (Fig. 1).
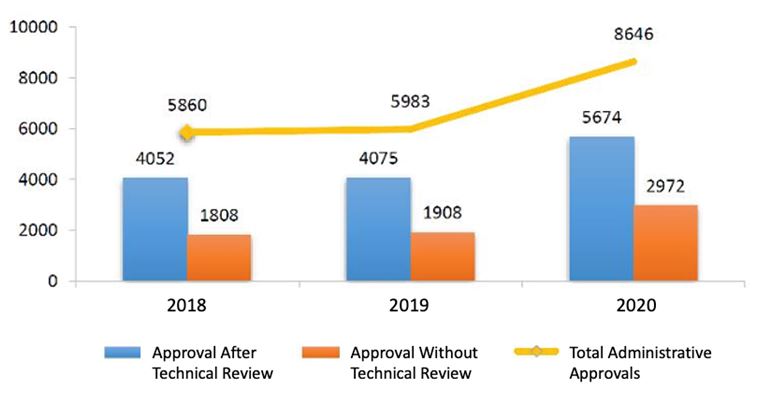
Fig. 1 Number of Administrative Approvals from 2018 to 2020.
2. Fast Track Review Pathways
CDE used several regulatory pathways to enhance efficiency as well as expedite drug development and approval in 2020.
According to the Provisions for Drug Registration (State Administration for Market Regulation [2020] Decree No. 27), the fast track review pathways are:
- Specialized pathway,
- Breakthrough therapy,
- Conditional approval,
- Priority review.
These pathways use a range of approaches, including:
- More interactions between CDE reviewers and drug developers,
- Greater application process flexibility,
- Shortened timelines for review and approval.
During the COVID-19 pandemic, fast track review pathways advanced patient treatments in approving drugs for unmet clinical needs and significant improvements on medical care. This better protected the health of the Chinese public.
Specialized Pathway
A drug reviewed by the Specialized Pathway is used for the treatment or prevention of pandemic disease as part of a declared public health emergency.
The drug review is expedited with:
- Increased flexibility:
- Accelerated application acceptance,
- Parallel proceeding of review,
- On-site inspection/audit,
- Product testing.
The drug can only be used in a specified period and is based on its medical need for controlling that pandemic disease.
During the COVID-19 pandemic, CDE reviewed 59 chemical drugs, biological products, and traditional Chinese medicines by the specialized pathway in 2020.
Among these:
- 1 novel drug (i.e., COVID-19 Vaccine (Vero Cell) Inactivated) was approved under controlled conditions,
- 53 novel drugs were approved for clinical trials,
- 5 listed drugs were approved to include a new indication of treating coronavirus.
2.2.Breakthrough Therapy
Breakthrough Therapy is a drug for a serious or life-threatening disease for which there is an unmet clinical need or for which there is preliminary clinical evidence demonstrating that the drug may have a substantial clinical improvement over other available therapies.
An application can be submitted to CDE in Phase I or II clinical trial for a drug to be designated as a Breakthrough Therapy.
Once accepted, a breakthrough therapy designation will have the following features:
- Priority of advisory communication and meetings with CDE as well as more intensive CDE guidance on drug development.
- Priority review with shortened approval timeline.
- Priority of on-site inspection/audit and product testing.
In 2020, CDE designated 24 of the 147 applied drugs (16%) as breakthrough therapies.
2.3. Conditional Approval
A drug receives Conditional Approval if there is an urgent medical need for the drug to treat a serious, life-threatening, or public health emergency disease.
The drug can result in having surrogate endpoints, intermediate endpoints, or early clinical data as to efficacy measures.
If the clinical study demonstrates that the benefit of the drug overweighs its risk, it will be approved under controlled conditions.
In 2020, 15 novel drugs received conditional approvals from CDE and provided new therapies for:
- COVID-19 pandemic,
- Non-small cell lung cancer,
- Ovarian cancer and more.
2.4. Priority Review
A drug receives a Priority Review if CDE determines that the drug could potentially provide significant advances in medical care or serve previously unmet medical needs.
The drug is reviewed in an expedited timeline, within 130 workdays instead of the standard 200 workdays, and within 70 workdays for a drug already approved overseas to treat a rare or “orphan” disease.
219 drugs were designated Priority Review by CDE in 2020, among which 42 drugs were pediatric and rare disease drugs.
Including the backlog of priority reviews of the previous year, 217 drugs (of 121 categories) were approved in 2020, an increase of 51.7% compared to 2019.
The highlight examples were:
- Almonertinib mesylate tablets,
- Agalsidase,
- Concentrated injection,
- Mulberry twig alkaloids tablets.
Table 2 gives an overview of priority review and approval in 2020 and compares the number of approvals from 2016 to 2020.
|
REGISTRATION APPLICATION |
2016 |
|
2017 |
|
2018 |
|
2019 |
|
2020 |
|
|---|---|---|---|---|---|---|---|---|---|---|
|
|
NUMBER OF CATEGORIES |
RATIO |
NUMBER OF CATEGORIES |
RATIO |
NUMBER OF CATEGORIES |
RATIO |
NUMBER OF CATEGORIES |
RATIO |
NUMBER OF CATEGORIES |
RATIO |
| Novel Drug with substantial clinical advance | 0 | 14.30% | 33 | 66% | 39 | 47% | 40 | 48.80% | 36 | 29.80% |
| Parallel application | 0 | 0% | 4 | 8% | 14 | 16.90% | 7 | 8.50% | 30 | 24.80% |
| AIDS Drug | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 3 | 2.50% |
| Orphan designation for rare disease | 0 | 0% | 0 | 0% | 3 | 3.60% | 6 | 7.30% | 11 | 9.10% |
| Pediatric drug | 4 | 57.10% | 1 | 2% | 9 | 10.80% | 7 | 8.50% | 8 | 6.60% |
| Re-application after generic consistency evaluation | 0 | 0% | 0 | 0% | 5 | 6% | 8 | 9.80% | 20 | 16.50% |
| Major national R&D program drug | 0 | 0% | 0 | 0% | 0 | 0% | 5 | 6.10% | 9 | 7.40% |
| Generic of coming off-patient drug | 1 | 14% | 2 | 4% | 4 | 4.80% | 7 | 8.50% | 4 | 3.30% |
| Drug of urgent clinical need or of market shortage | 0 | 0% | 2 | 4% | 3 | 3.60% | 0 | 0% | 0 | 0.00% |
| First generic drug approval | 1 | 14.30% | 8 | 16% | 6 | 7.20% | 2 | 2.40% | 0 | 0.00% |
| IN TOTAL | 7 | 100% | 50 | 100% | 83 | 100% | 82 | 100% | 121 | 100% |
- Drugs/Pharmaceutical,
- Food and Feed products for China NMPA (Formerly CFDA).
- Regulatory approval including product registration for China Investigational New Drug (IND),
- China New Drug Application (NDA),
- China Drug Master File (DMF) for APIs,
- Excipients and Packaging Materials,
- ANDA,
- eCTD submission and others.








