From the second half of 2018, China CDE (Center for Drug Evaluation) has significantly speeded up its review and approval process of imported drug, which can be considered as the result of the national faster imported drug approval policy. According to the statistics of the first quarter of 2019, the number of acceptances and the number of approvals for imported drug applications continued to grow on the basis of the fourth quarter of 2018.
Acceptance of imported drug applications:
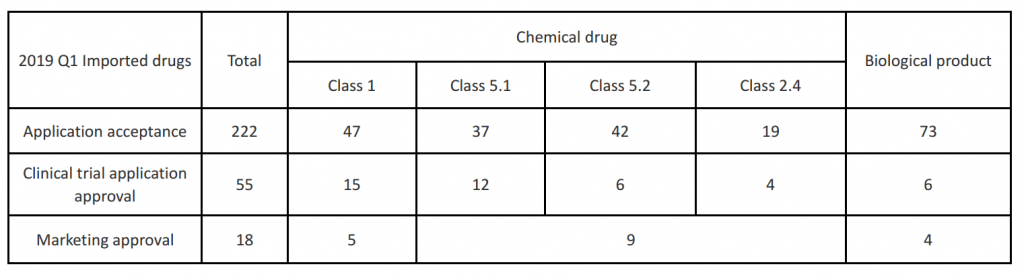
In the first quarter of 2019, China CDE accepted a total of 222 applications for imported drugs. Among them, the number of applications for therapeutic biological products was the highest, reaching 73 acceptance; the second was the new drug of Class 1, with 47 acceptance; The Class 5.1 and Class 5.2 drugs were basically the same, with 37 and 42 acceptance respectively; Class 2.4 drugs were the least, with only 19 acceptance.
Among the 222 imported drug acceptance, 138 of them are clinical applications, and the other 80 are marketing applications. And in all the drug varieties for marketing application, 6 of them are put in the priority review track of CDE: Nivolumab injection (Bristol-Myers Squibb), Adalimumab injection (AbbVie), Patezumab injection (Roche), Dabrafenib capsule (Novartis), Trametinib tablets (Novartis) and Pralatrexate injection (Mundipharma).
Imported drug clinical trial application approval:
In the first quarter of 2019, there are total 55 imported drug clinical trial applications approved by China CDE, including 15 of Class 1 new drugs, 12 of Class 5.1 drugs, 6 of Class 5.2 drugs, 4 of Class 2.4 drugs and 6 of biological products.
Marketing approval of imported drugs:
In the first quarter of 2019, the total marketing approval number of imported drugs is 18, including 10 drug varieties. And three of them were approved with priority review process as urgently needed drugs: Evolocumab Injection (Amgen), Spinraza injection (Biogen) and Dulaglutide Injection (Eli Lily). For these three drugs it costs 6-8 months in average from CDE accepting the application to the market approval being issued by NMPA.