For Drug master filing (DMF) in China, there is a new guideline released by China’s NMPA (Formerly CFDA) in 2019 for the scope of active pharmaceutical ingredients (APIs), excipients and packaging materials. Similar to the systems of US DMF, Canadian DMF and EU CEP, the new guideline helps protect manufacturers from disclosing confidential information to their distributors and specifies a more direct route to communicating with China health authorities, e.g., Center for drug evaluation (CDE).
Applicants who already have US DMF, Canadian DMF and EU CEP will benefit from overlapping data, although they’re differences in local data requirements.
The new DMF filing system in China helps protect the supplier from disclosing unnecessary confidential data to the drug applicant which enables a direct path to exchange communication with China’s health authority namely Centre for drug evaluation (CDE).
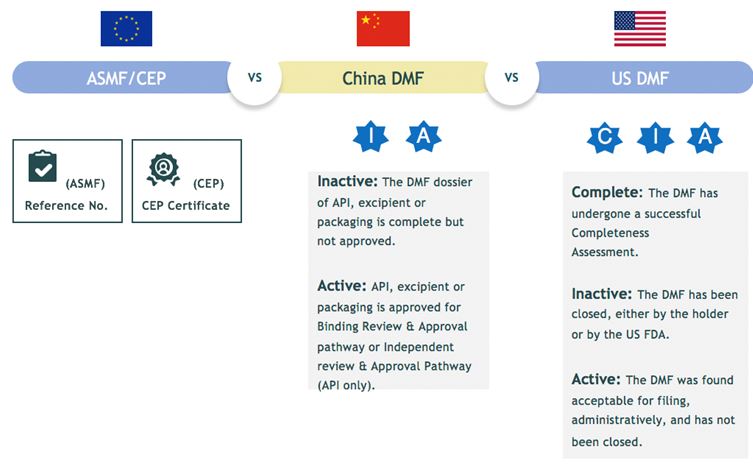
What are inactive and active DMF numbers in China?
The applicant prepares China DMF dossier and file application to CDE for completeness review for allocation of DMF filing number with a status of “I” for “Inactive”.
Upon drug product registration, for example ANDA or new drug application; CDE will refer to the inactive DMF filing number and review the DMF dossier together with the drug dossier and then activate the DMF filing number shown as “A” for active. The “I” and “A” status will officially display on CDE database.
Steps to File DMF in China
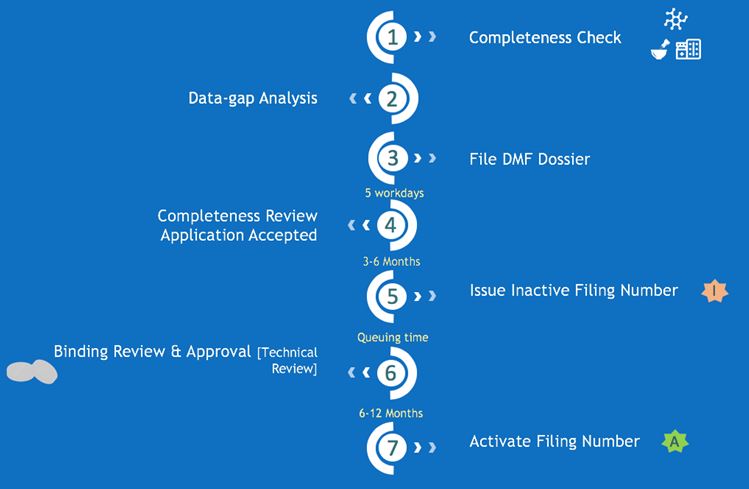
The 7 steps for DMF filing process to obtain inactive and active DMF filing number is displayed in the flow graph above and correspondingly explained below:
The general steps for DMF filing include obtaining inactive and active filing number described below:
Step 1: Check material completeness to identify if the materials are generally complete.
Step 2: Conduct a more detailed data-gap analysis, providing guidance on supplementing missing data according to China CDE requirements.
Step 3: Prepare a DMF dossier in Chinese and file to CDE.
Step 4: CDE reviews dossier for completeness and feedback in 1 to 2 weeks.
Step 5: CDE issues the Inactive filing number in around 3-6 months counting from step 2 data gap analysis.
Step 6: CDE performs technical review. The queuing time at CDE may differ depending on the actual situation. It was slightly longer due to Covid-19 but now recovered back to normal processing times.
Step 7: CDE usually takes from start to finish 6-12 months for the technical review and will activate the DMF filing number If the finished product has already been approved.
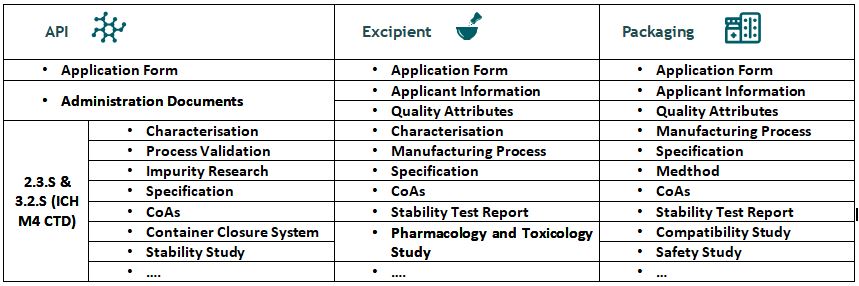
Checklist for DMF filing in China
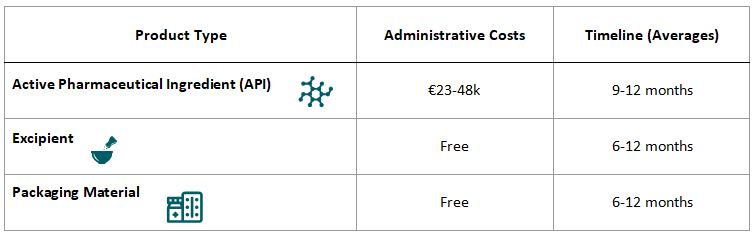
Costs & Timelines for DMF in China
China DMF Compared with US DMF, EU ASMF & CEP
Authorised Agent in China
Accestra Consulting has successfully helped overseas manufacturers to file drug master files (DMF) in China. We can be your trusted appointed agent by issuing us a letter of authorisation (LoA).
- Find out more about China Drug Master file (DMF) Regulations and standards
We would be more than happy to arrange a teleconference call you share an overview of the China DMF process, requirements, costs and timelines. Email us to arrange a date and time to call (Info@accestra.com).