Part 4. On-site/Distant Inspection and Advisory Meetings
Table of contents1. Drug GMP Inspection. |
Drug review and approval challenges in 2020 included overcoming difficulties in completing facility inspection, conducting remote advisory meetings, and facilitating communications through a variety of tools to help advance drug development and accelerate approval.
As a follow-up on part 3 of the Drug Evaluation Report 2020, part 4 gives an overview of on-site/distant GMP/GCP/GLP inspection, advisory meetings, and technical issue communication pathways, including phone calls, e-mails, and e-platform services by CDE..
|
1. Drug GMP Inspection
To assess the compliance of a site with the GMP/GCP/GLP of China, the Center of Drug Evaluation (CDE) requests the Center for Food and Drug Inspection (CFDI) for facility inspection when information, such as high-risk products, quality complaints, adverse events or data from an application dossier or other sources, indicate that potential problems or risks exist with a drug product (“For-cause” Inspection). For any application (NDA or ANDA) submitted after July 1st, 2020, a notice of on-site inspection is issued by CDE within 40 workdays after application acceptance if a “cause” warrants a timely investigation of that product. The notice and any other Information to guide the preparation for on-site inspection can be found on the e-platform of CDE through the “Gateway for Applicant”.
1.1 On-site Inspection
In 2020, CDE requested 1,235 on-site inspections, including 792 GMP inspections, 439 GCP inspections, and 4 GLP inspections. Including the backlog of previous year, 818 on-site inspections were completed by CFDI, correspondingly, 449 GMP reports, 363 GCP reports and 6 GLP reports were submitted to CDE.
1.2 Distant Inspection
Under the international impact of COVID-19 pandemic, overseas on-site inspections were not conducted for a number of reasons such as travel restrictions or risk to health. In these circumstances, distant inspections can represent a suitable means of determining GMP/GCP/GLP compliance based on documents and interviews while supported by IT technologies. In November 2020, CFDI conducted distant inspections for two drug products produced outside of China, the outcome of which was basically in line with the principles of on-site inspection. Nevertheless, on-site inspections should still be conducted once circumstances permit.
2. CDE Advisory Meetings and Communications
Advisory meetings and communications are activities of discussing crucial scientific, technical, and policy issues which are not specified in the guidelines for drug development and review of drug registration. They can be proposed by the applicant of drug registration or requested by CDE if clarifications are required during application review. To provide better guidance on these activities, CDE revised and NMPA issued the Provisions for Communications about Drug Development and Technical Review (NMPA [2020] Decree No.48) in 2020. To expedite drug review and approval, CDE took the initiative and newly established e-platform communication services for technical Q&As both before and after a deficiency notification letter is issued for a drug.
2.1 Overview
In the new model of communication, 3,229 applications were received in 2020 for advisory meetings with an increase of 22.6% comparing to 2019 and among which 2,451 applications were approved. On the e-platform of CDE, 20,285 questions were received about general technical issues in 2020 with an increase of 22.4%. In addition, more than ten thousand of phone calls and e-mails were received and answered. To compare the number of advisory meetings and communications between the last years, please see the diagrams below (Fig. 1 and 2).
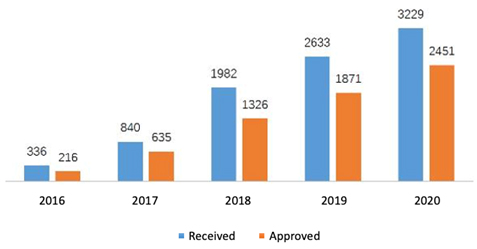
Fig. 1 Number of Applications for Advisory Meetings from 2016 to 2020
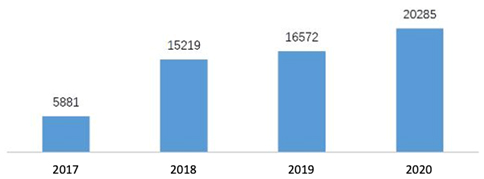
Fig. 2 Number of General Technical Issue Q&As from 2017 to 2020: the e-platform of general technical issue Q&As has been in operation since 2017.
2.2 CDE Advisory Meetings
To improve the efficiency with which expert advice on crucial issues is obtained, CDE determines whether a formal conference is required for a drug or quick answers can be given in writing after receiving the application. If a formal conference is confirmed, it can be held as a teleconference, videoconference, or face-to-face (F2F) meeting. Among 2,451 applications approved for advisory meetings in 2020, 268 conferences were held, and the rest were replied in writing. Classify these meetings according to their topics, Class II meetings shared 76.42% of total approvals, among which pre-IND meetings were the most requested (37.49%). To learn the classification of advisory meetings, please see the overview of advisory meetings below for 2020 (Table 1).
Table 1. Overview of CDE Advisory Meeting Applications and Approvals in 2020
| Classification of CDE Advisory Meetings | Number of Applications | % | Number of Approvals | % | |
|---|---|---|---|---|---|
| Class I (Meetings for crucial safety issues in clinical trials or major technical issues in drug development of breakthrough therapies) | 202 | 6.26% | 138 | 5.63% | |
| Class II | Pre-IND Meeting | 1,250 | 38.71% | 919 | 37.49% |
| IND Meeting | 228 | 7.06% | 171 | 6.98% | |
| End of Phase I Trial Meeting | 231 | 7.15% | 213 | 8.69% | |
| End of Phase II Trial Meeting | 241 | 7.46% | 188 | 7.67% | |
| Pre-NDA Meeting | 417 | 12.91% | 324 | 13.22% | |
| NDA Meeting | 71 | 2.20% | 45 | 1.84% | |
| Generic Consistency Evaluation Meeting | 1 | 0.03% | 3 | 0.12% | |
| Complex Generic ANDA Meeting | 17 | 0.53% | 10 | 0.41% | |
| Class III (Other meetings) | 571 | 17.68% | 440 | 17.95% | |
| In Total | 3,229 | 100% | 2,451 | 100% | |
Note: Some of the advisory meetings approved in 2020 were backlog of the previous year.
2.3 Fast-track Review Meetings
Under the COVID-19 public health emergency, CDE approved many new drug therapies in 2020 for patients suffering from the pandemic through fast-track review pathways. To shorten the timeline for approval, CDE encouraged drug innovators to file applications while development and study of drugs were ongoing. Within 24 hours after an application is received, CDE communicated with the applicant proactively about any crucial scientific matters and the feasibility of approval. For any new technological challenges, CDE invited independent experts for advisory meetings and consultations to obtain advice and discuss solutions. The decisions made on these approvals were further included in the technical guidelines to support drug development and speed new therapies to market.
2.4 Technical Issue Communications
Except for the advisory meetings, applicants of drug registration can also ask questions about general technical issues on the e-platform of CDE. Classify the questions received according to their topics, issues about application acceptance and Active Pharmaceutical Ingredient (API)/Excipient/Packaging were the most asked. Classify the questions received according to drug categories, issues about chemical drugs were the most asked. For more details, please see the overview of general technical issue Q&As below for 2020 (Table 2).
Table 2. Number of Q&As for General Technical Issues in 2020
|
CLASSIFICATION OF Q&As |
API/ EXCIPIENT / PACKING |
CHEMICAL DRUG |
BIOLOGICAL PRODUCT |
TRADITIONAL CHINESE MEDICINE & NATURAL MEDICINE |
OTHERS |
IN TOTAL |
|---|---|---|---|---|---|---|
| Application Acceptance | 315 | 2,396 | 790 | 141 | 397 | 4,39 |
| API/ Excipient / Packing | 2,764 | 1,055 | 85 | 30 | 18 | 3,952 |
| Techical Review – CMC | 47 | 1,077 | 482 | 46 | 18 | 1,670 |
| Genric Consistency Evaluation | 10 | 1,258 | 7 | 2 | 35 | 1,312 |
| Technical Review – Clinical Trials | 0 | 824 | 335 | 59 | 37 | 1,255 |
| Technical Review – Regulatory Compliance | 47 | 668 | 216 | 64 | 47 | 1,042 |
| Material Supplements for Deficiency Letter | 163 | 718 | 87 | 28 | 14 | 1,010 |
| Guidelines | 33 | 519 | 164 | 58 | 118 | 892 |
| CDE e-Platform | 225 | 151 | 29 | 4 | 52 | 461 |
| Technical Review – Pharmacology, Toxicology | 0 | 158 | 113 | 16 | 3 | 290 |
| Technical Review – Statistics / Clinical Pharmacology | 0 | 111 | 34 | 4 | 3 | 152 |
| Others | 197 | 2,331 | 607 | 175 | 381 | 3,691 |
| In Total | 3,804 | 11,338 | 3,381 | 636 | 1,125 | 20,285 |
Accestra Consulting Provides China Regulatory Affairs Outsourcing for:
- Drugs/Pharmaceutical,
- Food and Feed products for China NMPA (Formerly CFDA).
- Regulatory approval including product registration for China Investigational New Drug (IND),
- China New Drug Application (NDA),
- China Drug Master File (DMF) for APIs,
- Excipients and Packaging Materials,
- ANDA,
- eCTD submission and others.








